正在加载图片...
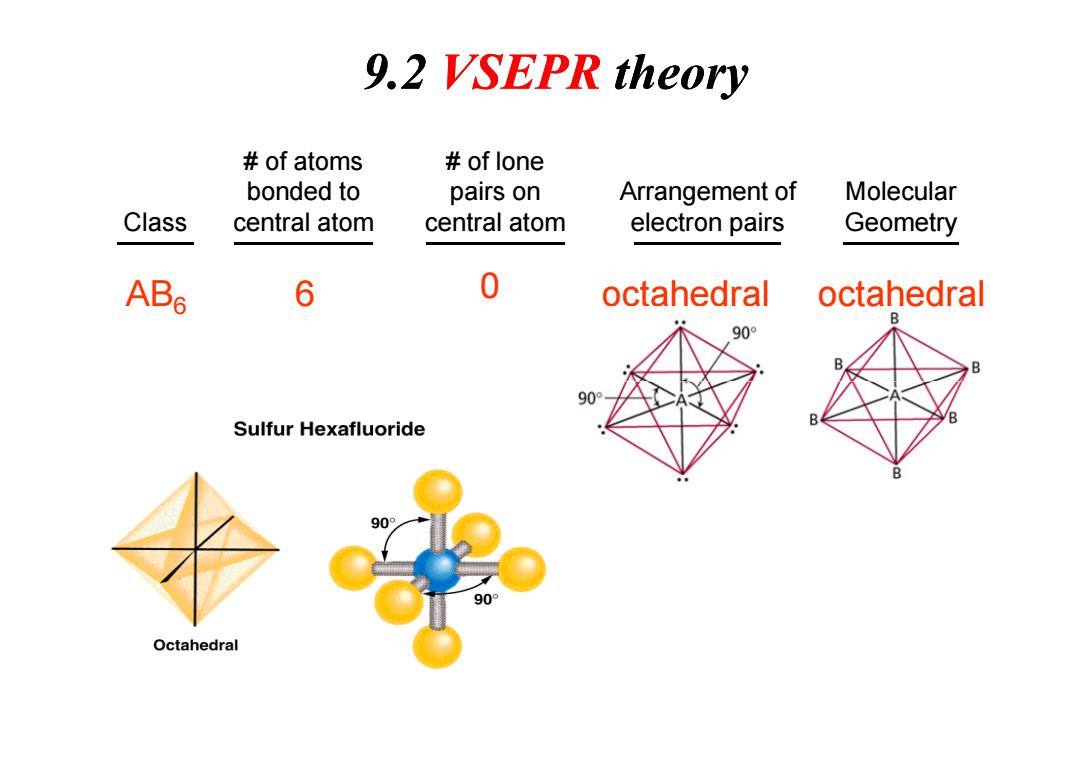
9.2 VSEPR theory of atoms of lone bonded to pairs on Arrangement of Molecular Class central atom central atom electron pairs Geometry ABs 6 0 octahedral octahedral 90° 90 B Sulfur Hexafluoride 90° 90° OctahedralClass # of atoms bonded to central atom # of lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB6 6 0 octahedral octahedral 9.2 VSEPR theory