正在加载图片...
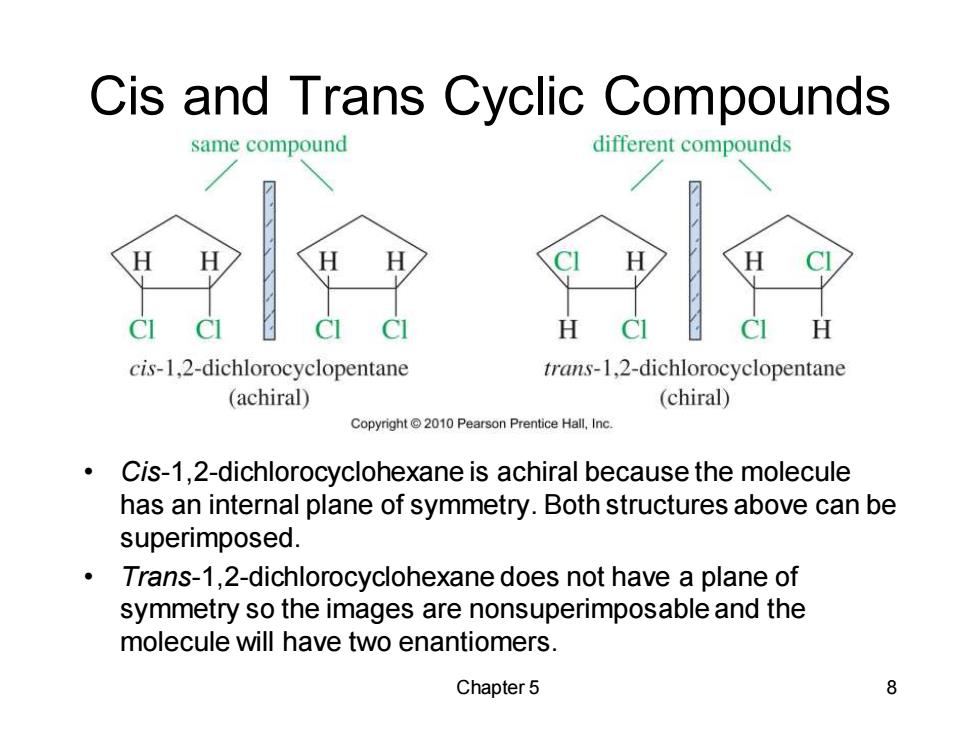
Cis and Trans Cyclic Compounds same compound different compounds ci CI H cis-1,2-dichlorocyclopentane trans-1,2-dichlorocyclopentane (achiral) (chiral) Copyright 2010 Pearson Prentice Hall,Inc. Cis-1,2-dichlorocyclohexane is achiral because the molecule has an internal plane of symmetry.Both structures above can be superimposed. Trans-1,2-dichlorocyclohexane does not have a plane of symmetry so the images are nonsuperimposable and the molecule will have two enantiomers. Chapter 5 8 Chapter 5 8 Cis and Trans Cyclic Compounds • Cis-1,2-dichlorocyclohexane is achiral because the molecule has an internal plane of symmetry. Both structures above can be superimposed. • Trans-1,2-dichlorocyclohexane does not have a plane of symmetry so the images are nonsuperimposable and the molecule will have two enantiomers