正在加载图片...
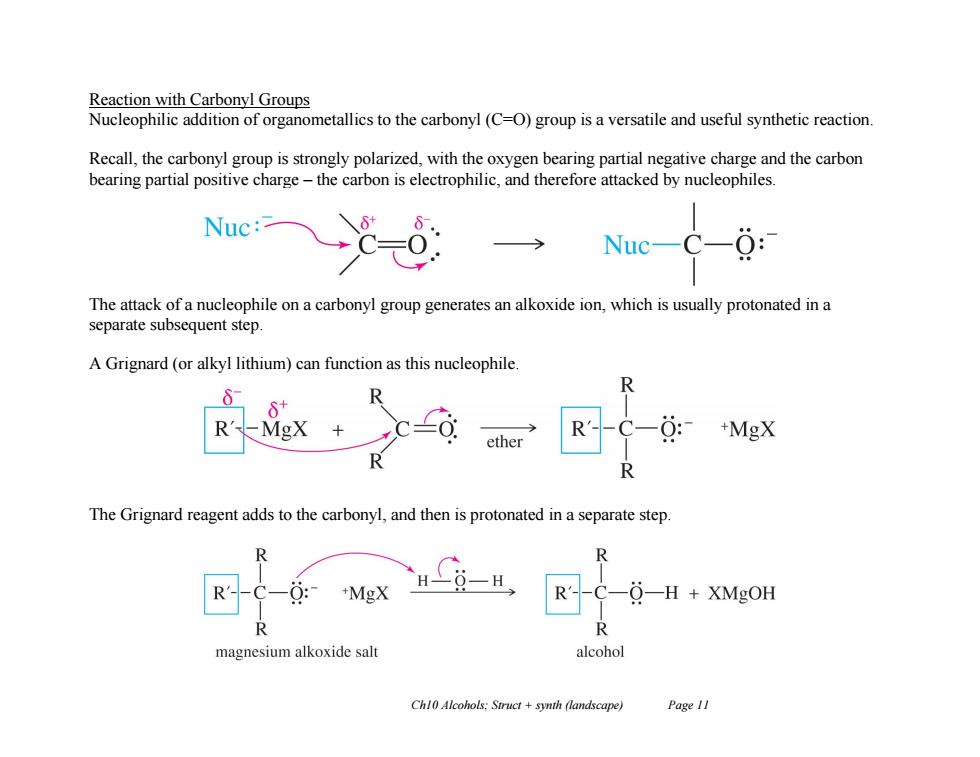
Reaction with Carbonyl Groups Nucleophilic addition of organometallics to the carbonyl(C=O)group is a versatile and useful synthetic reaction Recall,the carbonyl group is strongly polarized,with the oxygen bearing partial negative charge and the carbon bearing partial positive charge-the carbon is electrophilic,and therefore attacked by nucleophiles. Nuc: Nuc-C-6: The attack of a nucleophile on a carbonyl group generates an alkoxide ion,which is usually protonated in a separate subsequent step. A Grignard(or alkyl lithium)can function as this nucleophile. R -MgX ether +MgX The Grignard reagent adds to the carbonyl,and then is protonated in a separate step. R +MgX O-H XMgOH R magnesium alkoxide salt alcohol Ch10 Alcohols:Struct+synth (landscape) Page 11 Ch10 Alcohols; Struct + synth (landscape) Page 11 Reaction with Carbonyl Groups Nucleophilic addition of organometallics to the carbonyl (C=O) group is a versatile and useful synthetic reaction. Recall, the carbonyl group is strongly polarized, with the oxygen bearing partial negative charge and the carbon bearing partial positive charge – the carbon is electrophilic, and therefore attacked by nucleophiles. The attack of a nucleophile on a carbonyl group generates an alkoxide ion, which is usually protonated in a separate subsequent step. A Grignard (or alkyl lithium) can function as this nucleophile. The Grignard reagent adds to the carbonyl, and then is protonated in a separate step