正在加载图片...
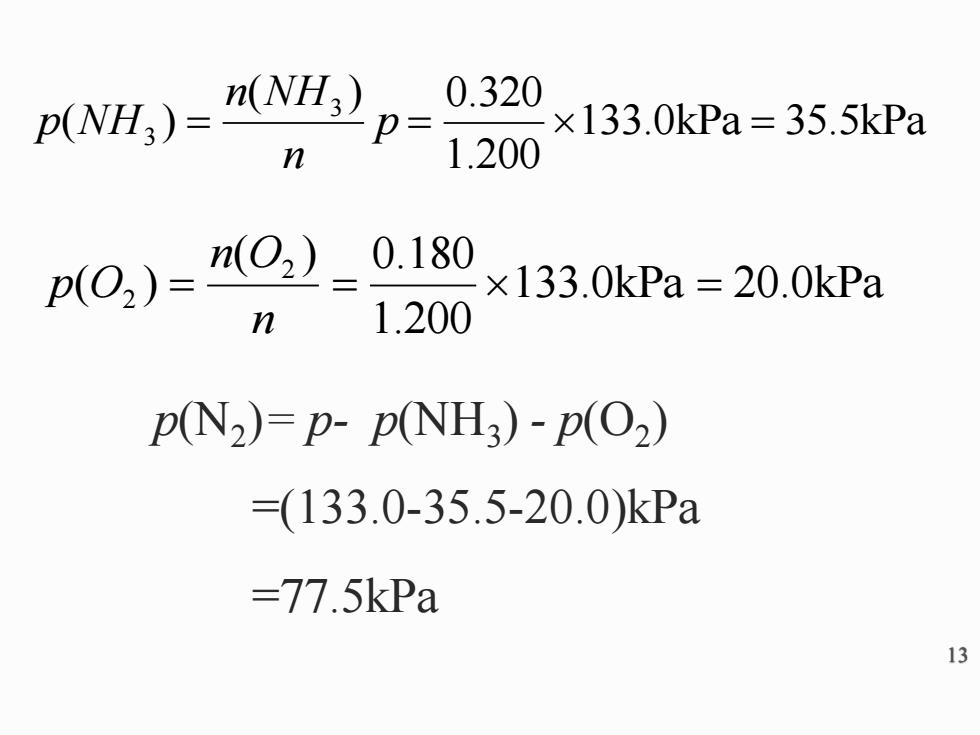
n(NH3) 0.320 p(NH;)= p= ×133.0kPa=35.5kPa n 1.200 p()=2) 0.180 ×133.0kPa=20.0kPa n 1.200 p(N2)=p-p(NH3)-p(O2) =(133.0-35.5-20.0)kPa =77.5kPa 13 13 p(N2 )= p- p(NH3 ) - p(O2 ) =(133.0-35.5-20.0)kPa =77.5kPa 133.0kPa 20.0kPa 1.200 ( ) 0.180 ( ) 2 2 = = = n n O p O 133.0kPa 35.5kPa 1.200 ( ) 0.320 ( ) 3 3 = p = = n n NH p NH