正在加载图片...
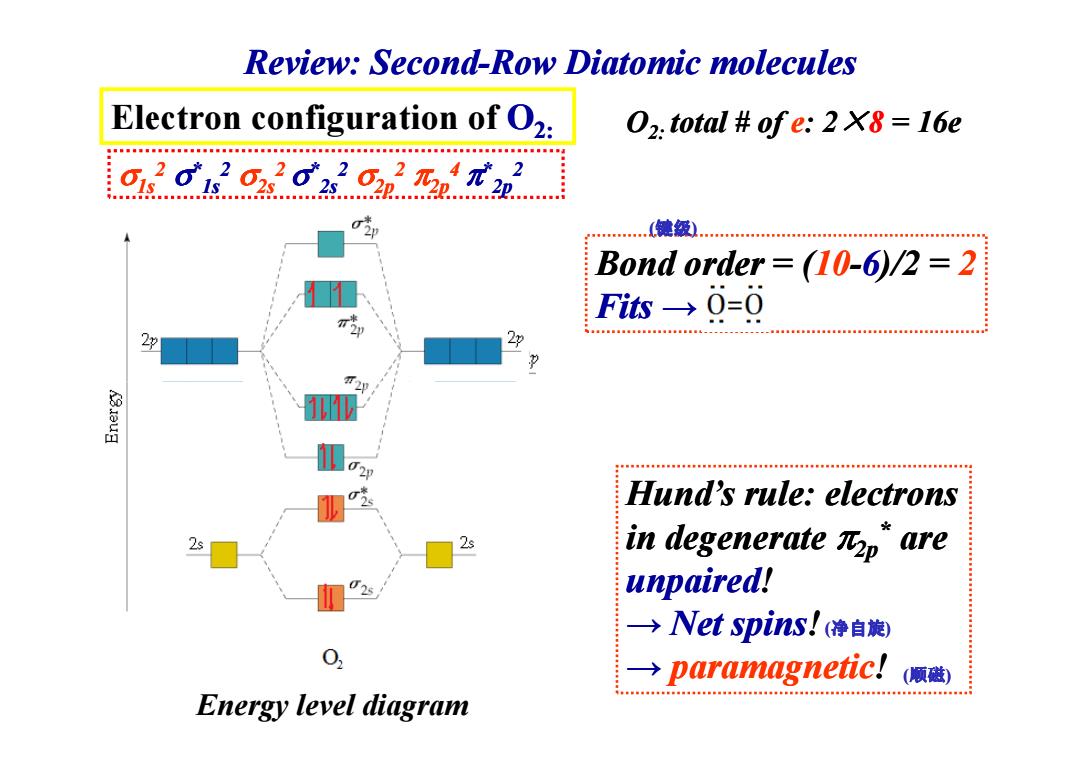
Review:Second-Row Diatomic molecules Electron configuration of O2: 02.total ofe:2 X8 16e 01 615 …(键级 Bond order (10-6)/2 =2 Fis→0=0 Hund's rule:electrons 2s in degenerate Rp"are unpaired! →Net spins!(净自旋) → paramagnetic! (顺磁) Energy level diagramElectron configuration of O2: σ1s2 σ*1s2 σ2s2 σ*2s2 σ2p2 π2p4 π*2p2 Bond order = (10-6)/2 = 2 Fits → O2: total # total # of e: 2 × 8 = 16e (键级) Review: Second Review: Second-Row Diatomic molecules Diatomic molecules Hund’s rule: electrons in degenerate π2p* are unpaired! → Net spins Net spins! → paramagnetic! Energy level diagram (净自旋) (顺磁)