正在加载图片...
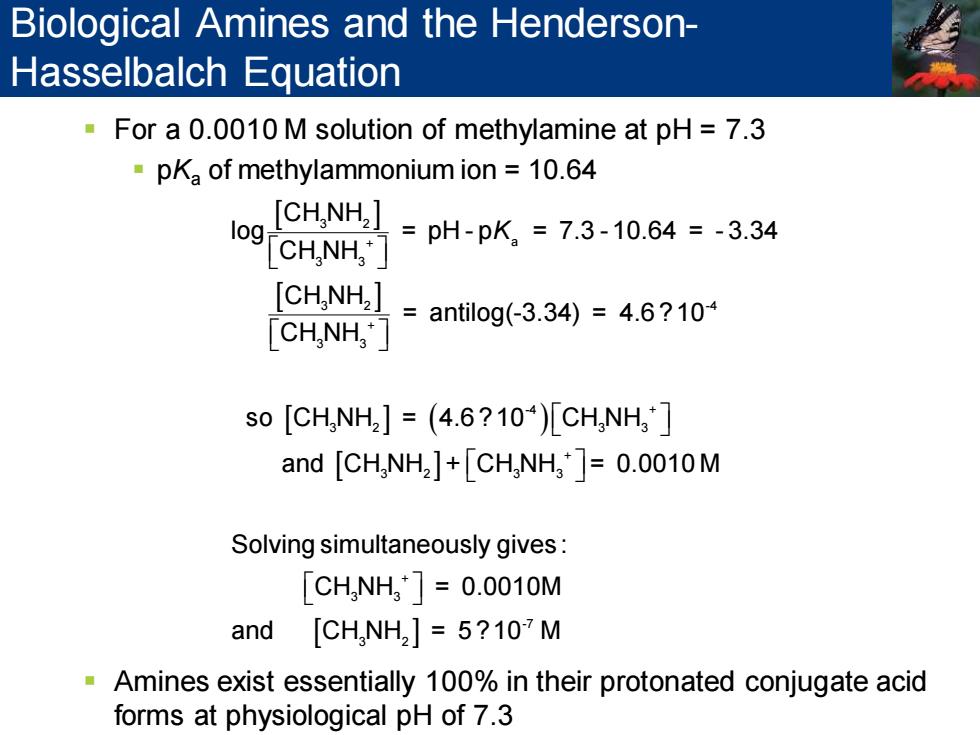
Biological Amines and the Henderson- Hasselbalch Equation For a 0.0010 M solution of methylamine at pH=7.3 pKa of methylammonium ion 10.64 [CHNH2] I9 CHNH] =pH-pK2=7.3-10.64=-3.34 [CHNH]=antilog(3.34)=4.6?10 CH,NH," so[CH,NH2]=(4.6?104)[CHNH3] and [CHNH2]+CH NH,=0.0010M Solving simultaneously gives: [CH,NH3]=0.0010M and [CH,NH2]5?107 M Amines exist essentially 100%in their protonated conjugate acid forms at physiological pH of 7.3 ▪ For a 0.0010 M solution of methylamine at pH = 7.3 ▪ pKa of methylammonium ion = 10.64 ▪ Amines exist essentially 100% in their protonated conjugate acid forms at physiological pH of 7.3 ( ) 3 2 + a 3 3 3 2 -4 + 3 3 -4 + 3 2 3 3 + 3 2 3 3 CH NH log = pH - p = 7.3 - 10.64 = - 3.34 CH NH CH NH = antilog(-3.34) = 4.6 ?10 CH NH so CH NH = 4.6 ?10 CH NH and CH NH + CH NH = 0.0010 M Sol K + 3 3 -7 3 2 ving simultaneously gives: CH NH = 0.0010M and CH NH = 5 ?10 M Biological Amines and the HendersonHasselbalch Equation