正在加载图片...
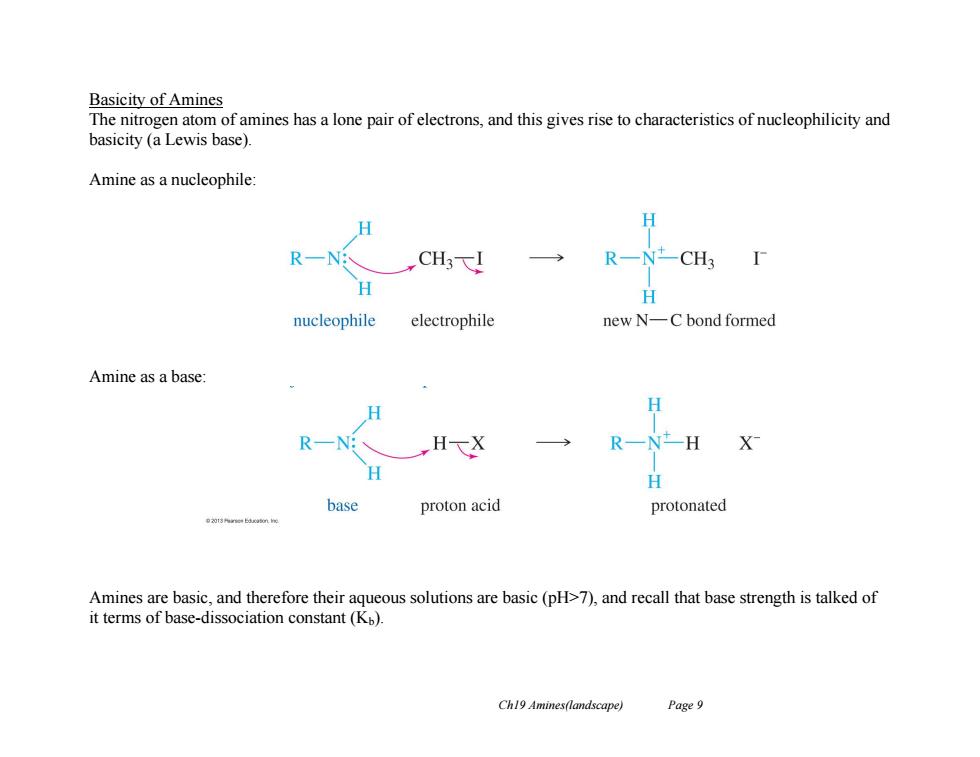
Basicity of Amines The nitrogen atom of amines has a lone pair of electrons,and this gives rise to characteristics of nucleophilicity and basicity (a Lewis base). Amine as a nucleophile H H R一NCH3F H nucleophile electrophile new N-C bond formed Amine as a base: H R一Nt- H X H base proton acid protonated Amines are basic,and therefore their aqueous solutions are basic (pH>7),and recall that base strength is talked of it terms of base-dissociation constant(Kp). Ch19 Amines(landscape) Page 9Ch19 Amines(landscape) Page 9 Basicity of Amines The nitrogen atom of amines has a lone pair of electrons, and this gives rise to characteristics of nucleophilicity and basicity (a Lewis base). Amine as a nucleophile: Amine as a base: Amines are basic, and therefore their aqueous solutions are basic (pH>7), and recall that base strength is talked of it terms of base-dissociation constant (Kb)