正在加载图片...
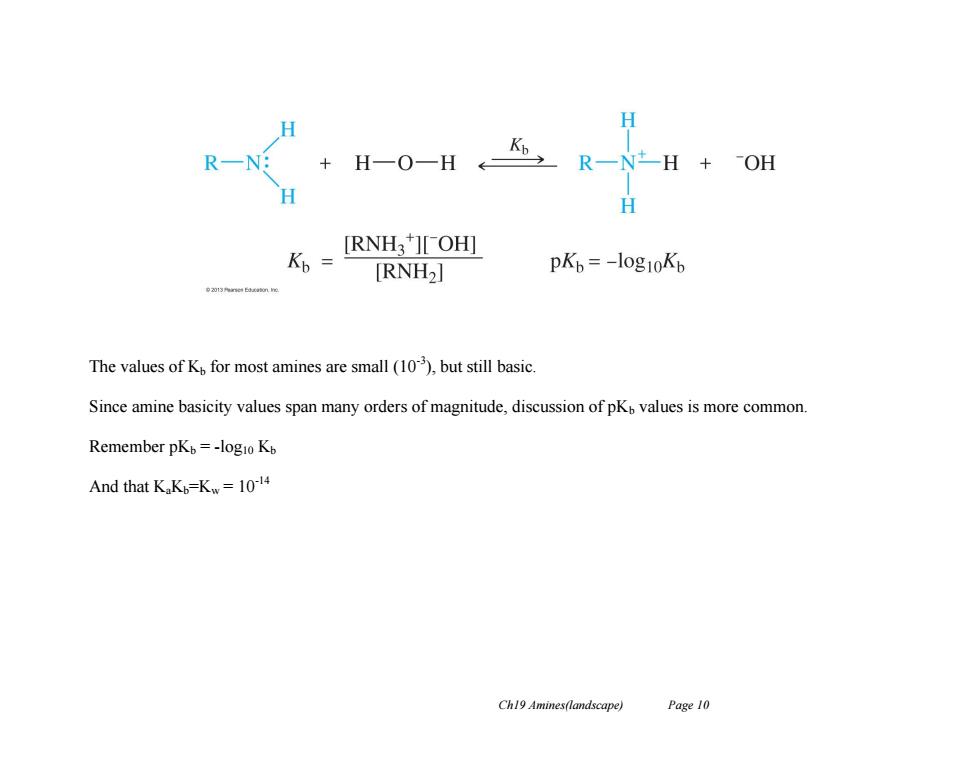
H R-N: H-0-HR-H+0附 + H K,= [RNH3][OH] [RNH2] pKb=-l0g10Kb The values of K for most amines are small (10),but still basic. Since amine basicity values span many orders of magnitude,discussion of pKo values is more common. Remember pKb=-logio Kb And that KaK=Kw=10-14 Ch19 Amines(landscape) Page 10 Ch19 Amines(landscape) Page 10 The values of Kb for most amines are small (10-3 ), but still basic. Since amine basicity values span many orders of magnitude, discussion of pKb values is more common. Remember pKb = -log10 Kb And that KaKb=Kw = 10-14