正在加载图片...
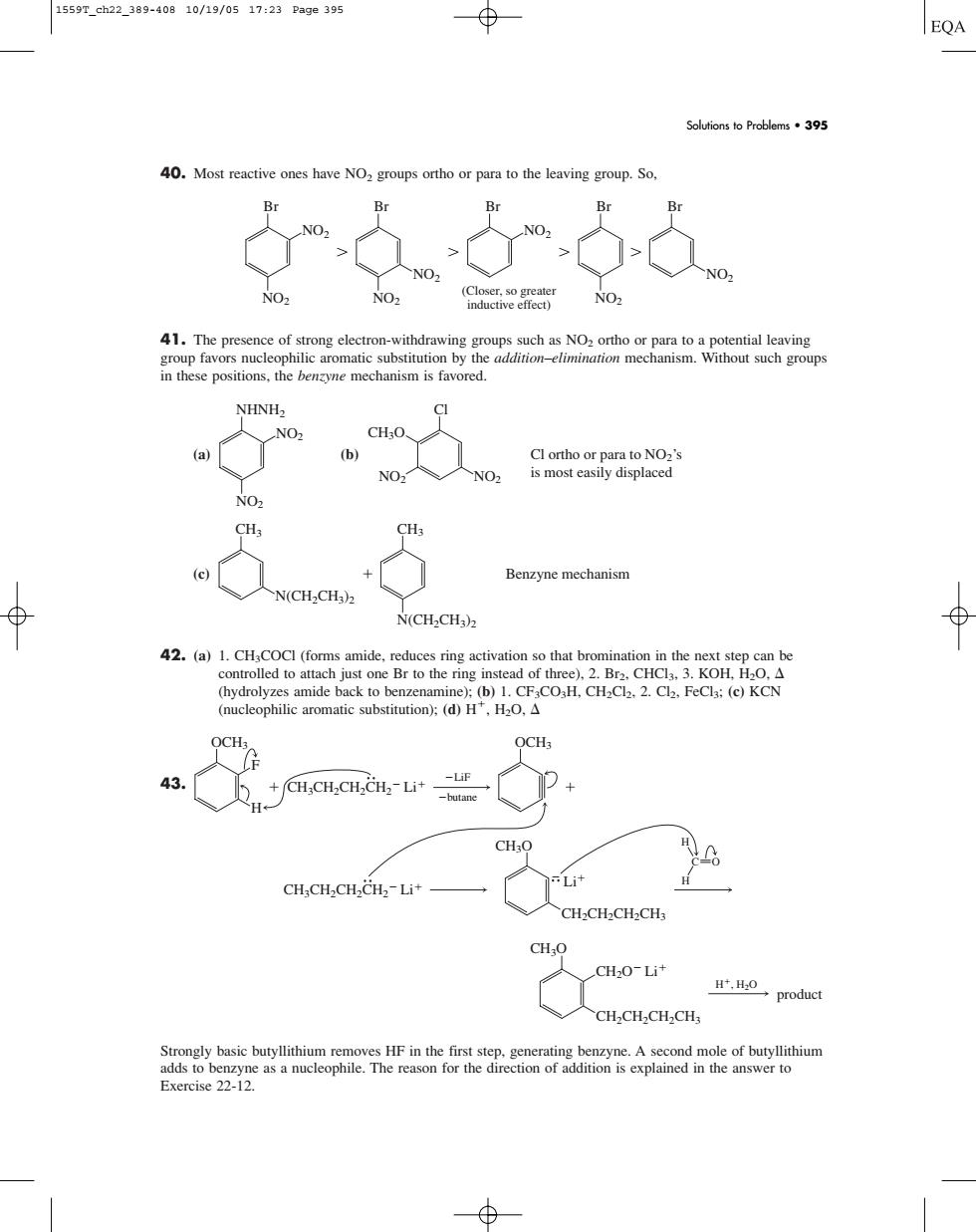
1559T_ch22_389-40810/19/0517:23Pa9e395 ⊕ EQA Solutions to Problems.395 40 ing group 41.The presence of strong electror CH:O. Cl ortho or para to no?'s No. is most easily displaced NO CH Benzyne mechanism N(CHCH N(CH2CH) 42.aCCCettmote2eatKoe mide metRmmmHoHad2a.aoka ,H0,△ CH,CH2CH2CH2-Li* 入CH,CH,CH,CH CH.O CH2O-Li* H.H0,product CH2CH2CH2CHs Strongly basic butyllithium s HE in the firs 40. Most reactive ones have NO2 groups ortho or para to the leaving group. So, 41. The presence of strong electron-withdrawing groups such as NO2 ortho or para to a potential leaving group favors nucleophilic aromatic substitution by the addition–elimination mechanism. Without such groups in these positions, the benzyne mechanism is favored. (a) (b) (c) 42. (a) 1. CH3COCl (forms amide, reduces ring activation so that bromination in the next step can be controlled to attach just one Br to the ring instead of three), 2. Br2, CHCl3, 3. KOH, H2O, (hydrolyzes amide back to benzenamine); (b) 1. CF3CO3H, CH2Cl2, 2. Cl2, FeCl3; (c) KCN (nucleophilic aromatic substitution); (d) H, H2O, 43. Strongly basic butyllithium removes HF in the first step, generating benzyne. A second mole of butyllithium adds to benzyne as a nucleophile. The reason for the direction of addition is explained in the answer to Exercise 22-12. CH3O CH2CH2CH2CH3 CH2O Li H, H2O product CH3O CH2CH2CH2CH3 CH3CH2CH2CH2 Li Li OCH3 OCH3 C O H H CH3CH2CH2CH2 Li LiF butane F H N(CH2CH3)2 CH3 N(CH2CH3)2 CH3 Benzyne mechanism NO2 CH3O NO2 Cl Cl ortho or para to NO2’s is most easily displaced NO2 NO2 NHNH2 NO2 NO2 Br NO2 Br NO2 NO2 Br NO2 Br NO2 Br (Closer, so greater inductive effect) Solutions to Problems • 395 1559T_ch22_389-408 10/19/05 17:23 Page 395���������������