正在加载图片...
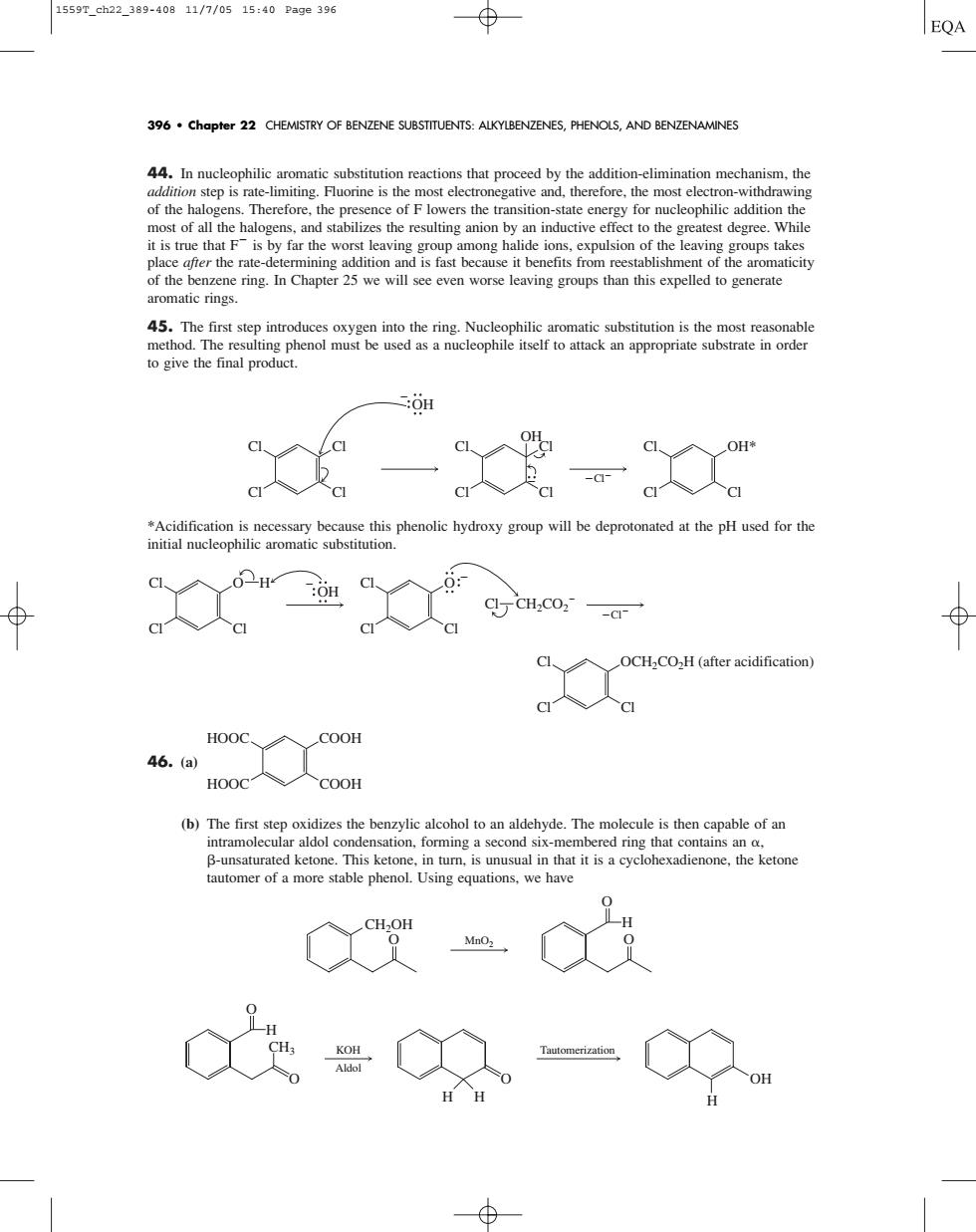
15597.ch22.389-40811/7/0515:40Page396 EQA 396 chapter 22 CHEMISTRY OF BENZENE SUBSTITUENTS:ALKYLBENZENES,PHENOLS,AND BENZENAMINES tion mechanism,the most of all the halogens.and stabilizes the resulting anion by an inductive effect to the greatest degree.While of the benzene ring.In Chapter 25 we will see even worse leaving groups than this expelled to generate aromatic rings. 45.The first step introd to give the final product. C:OH c人a OCHaCO2H(after acidification) 46.(a) 人COOH (b)The first step oxidizes the benzylic alcohol to an aldehyde.The molecule is then capable of an automer of a more stable phenol.Using equations,we have “ 及"44. In nucleophilic aromatic substitution reactions that proceed by the addition-elimination mechanism, the addition step is rate-limiting. Fluorine is the most electronegative and, therefore, the most electron-withdrawing of the halogens. Therefore, the presence of F lowers the transition-state energy for nucleophilic addition the most of all the halogens, and stabilizes the resulting anion by an inductive effect to the greatest degree. While it is true that F is by far the worst leaving group among halide ions, expulsion of the leaving groups takes place after the rate-determining addition and is fast because it benefits from reestablishment of the aromaticity of the benzene ring. In Chapter 25 we will see even worse leaving groups than this expelled to generate aromatic rings. 45. The first step introduces oxygen into the ring. Nucleophilic aromatic substitution is the most reasonable method. The resulting phenol must be used as a nucleophile itself to attack an appropriate substrate in order to give the final product. *Acidification is necessary because this phenolic hydroxy group will be deprotonated at the pH used for the initial nucleophilic aromatic substitution. 46. (a) (b) The first step oxidizes the benzylic alcohol to an aldehyde. The molecule is then capable of an intramolecular aldol condensation, forming a second six-membered ring that contains an , -unsaturated ketone. This ketone, in turn, is unusual in that it is a cyclohexadienone, the ketone tautomer of a more stable phenol. Using equations, we have H CH3 O O KOH Aldol Tautomerization O H H H OH CH2OH H O O O MnO2 COOH COOH HOOC HOOC Cl Cl Cl OCH2CO2H (after acidification) Cl Cl Cl Cl Cl Cl Cl O H Cl CH2CO2 OH O OH Cl Cl Cl Cl OH* Cl Cl Cl Cl Cl Cl Cl Cl OH 396 • Chapter 22 CHEMISTRY OF BENZENE SUBSTITUENTS: ALKYLBENZENES, PHENOLS, AND BENZENAMINES 1559T_ch22_389-408 11/7/05 15:40 Page 396��