正在加载图片...
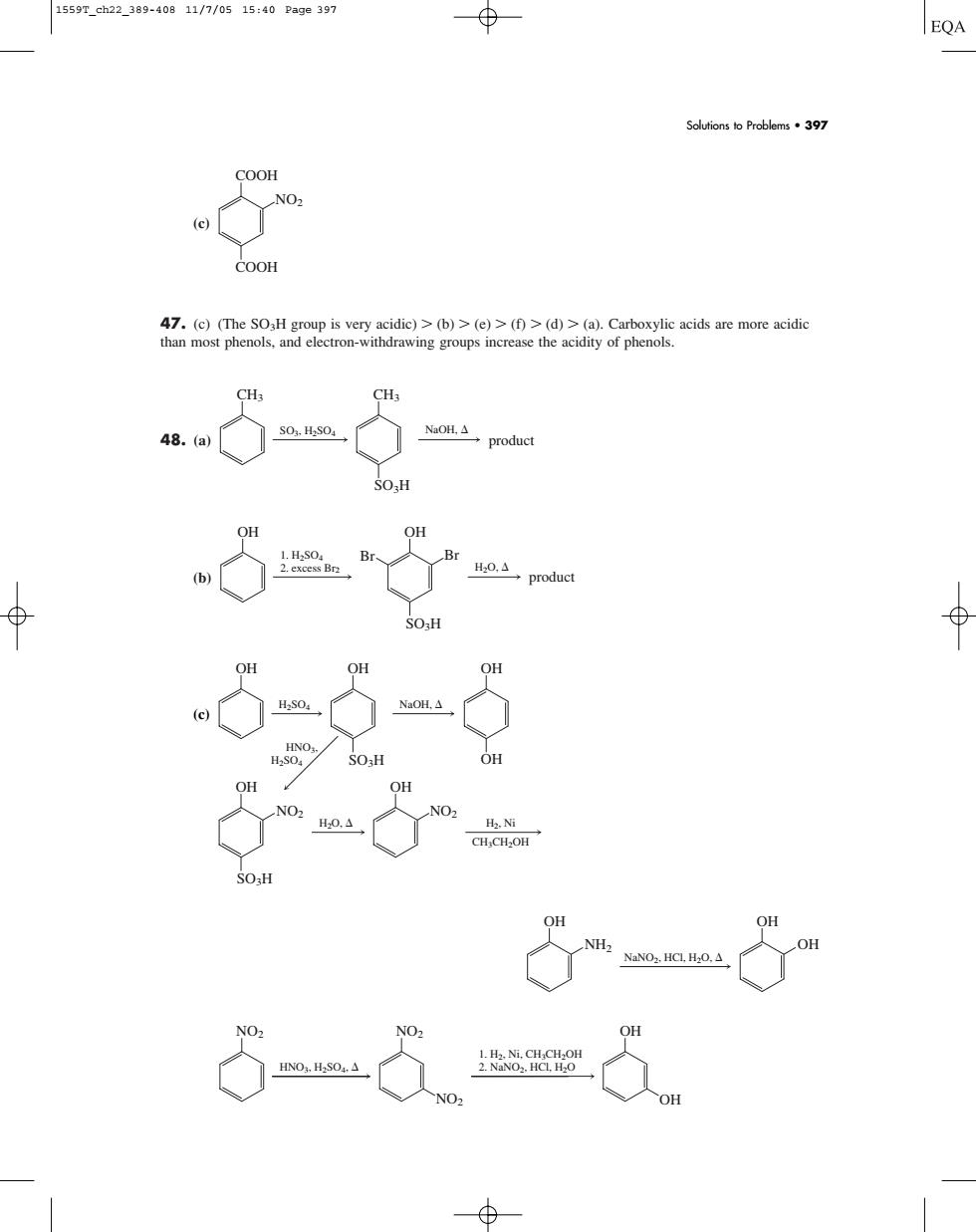
1559T_ch22_389-40811/7/0515:40Page397 EQA Soutionso Problems397 COOH COOH nost phenol wing groups increase the acidity of phenol 5ed O- OH OH OH NH. (c) 47. (c) (The SO3H group is very acidic) (b) (e) (f) (d) (a). Carboxylic acids are more acidic than most phenols, and electron-withdrawing groups increase the acidity of phenols. 48. (a) (b) product OH SO3H OH Br Br H2O, 1. H2SO4 2. excess Br2 CH3 CH3 SO3H SO3, H2SO4 NaOH, product COOH COOH NO2 Solutions to Problems • 397 (c) HNO3, H2SO4, 1. H2, Ni, CH3CH2OH 2. NaNO2, HCl, H2O NO2 NO2 NO2 OH OH NaNO2, HCl, H2O, OH OH OH NH2 OH OH OH OH SO3H NaOH, H2O, H2, Ni CH3CH2OH H2SO4 HNO3, H2SO4 OH NO2 SO3H OH NO2 1559T_ch22_389-408 11/7/05 15:40 Page 397