正在加载图片...
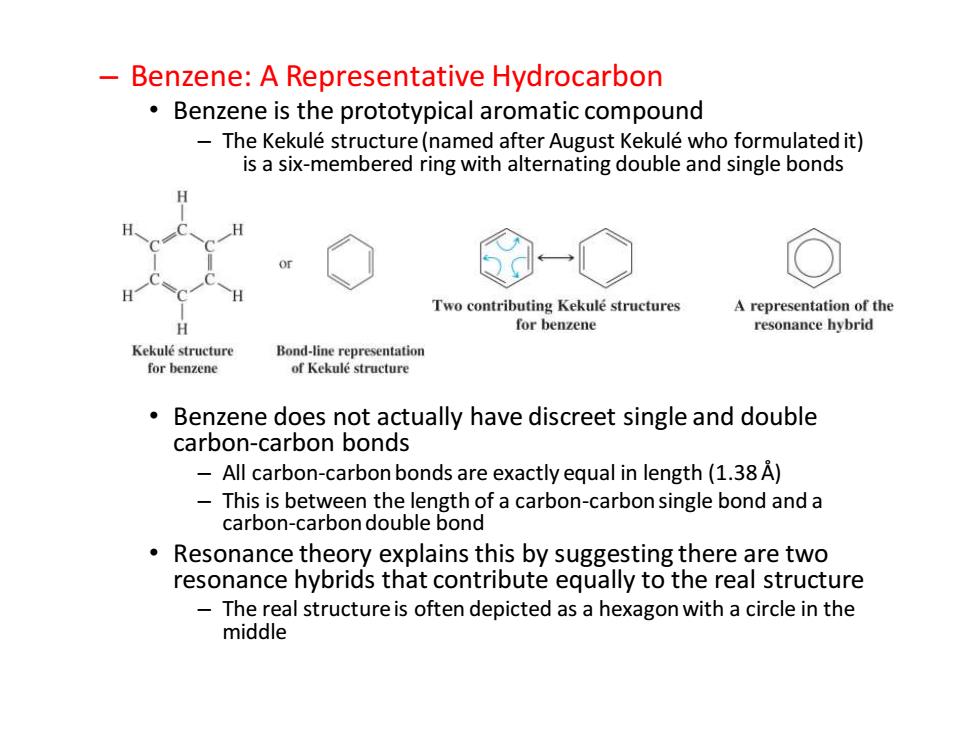
Benzene:A Representative Hydrocarbon Benzene is the prototypical aromatic compound The Kekule structure(named after August Kekule who formulated it) is a six-membered ring with alternating double and single bonds Two contributing Kekule structures A representation of the H for benzene resonance hybrid Kekule structure Bond-line representation for benzene of Kekule structure Benzene does not actually have discreet single and double carbon-carbon bonds 一 All carbon-carbon bonds are exactly equal in length(1.38 A) This is between the length of a carbon-carbon single bond and a carbon-carbon double bond Resonance theory explains this by suggesting there are two resonance hybrids that contribute equally to the real structure The real structure is often depicted as a hexagon with a circle in the middle– Benzene: A Representative Hydrocarbon • Benzene is the prototypical aromatic compound – The Kekulé structure (named after August Kekulé who formulated it) is a six-membered ring with alternating double and single bonds • Benzene does not actually have discreet single and double carbon-carbon bonds – All carbon-carbon bonds are exactly equal in length (1.38 Å) – This is between the length of a carbon-carbon single bond and a carbon-carbon double bond • Resonance theory explains this by suggesting there are two resonance hybrids that contribute equally to the real structure – The real structure is often depicted as a hexagon with a circle in the middle