正在加载图片...
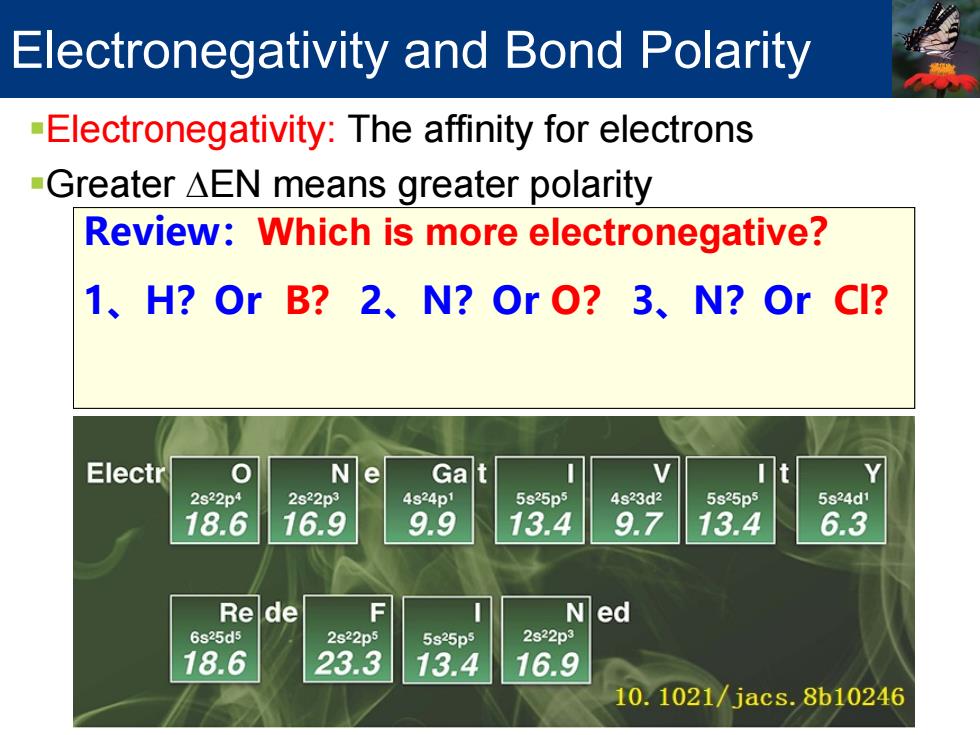
Electronegativity and Bond Polarity -Electronegativity:The affinity for electrons -Greater AEN means greater polarity Review:Which is more electronegative? 1、H?OrB?2、N?OrO?3、N?OrCI? Electr 0 Ne Gat 2s22p4 2s22p3 4s24p1 5s25p5 4s23d2 5s25p5 5s24d1 18.6 16.9 9.9 13.4 9.7 13.4 6.3 Rede F Ned 6s25d5 2s22p5 5s25p5 2s22p3 18.6 23.3 13.4 16.9 10.1021/jacs.8b10246Electronegativity: H: 2.1 B: 2.0 C: 2.5 N: 3.0 O: 3.5 F: 4.0 Cl: 3.0 Br: 2.8 I: 2.5 Li: 1.0 Mg: 1.2 Review:Which is more electronegative? 1、H?Or B? 2、N?Or O? 3、N?Or Cl? Electronegativity and Bond Polarity Electronegativity: The affinity for electrons Greater ∆EN means greater polarity