正在加载图片...
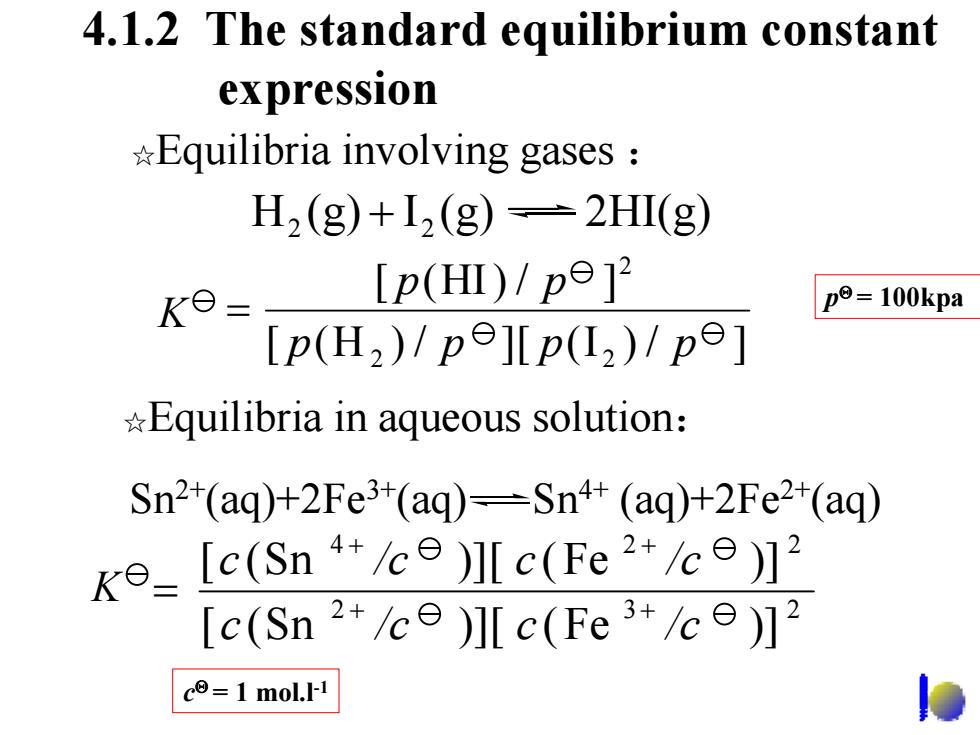
4.1.2 The standard equilibrium constant expression Equilibria involving gases H2(g)+I2(g)=2HI(g) [p(HⅡ)/pe]2 K8= p=100kpa [p(H2)1pe][p(L2)/pe] *Equilibria in aqueous solution: Sn2*(aq)+2Fe3(aq)=Sn4+(aq)+2Fe2*(aq) ke [c(Sn "le)ll c(Fe)2 [c(Sn 2+/c )]Ic(Fe 3+/ce)]2 c=1 mol.I-1 4.1.2 The standard equilibrium constant expression ☆Equilibria in aqueous solution : H (g) I (g) 2HI(g) 2 + 2 ☆Equilibria involving gases : Sn2+(aq)+2Fe3+(aq) Sn4+ (aq)+2Fe2+(aq) [ ( H ) / ][ ( I ) / ] [ (HI ) / ] 2 2 2 p p p p p p K = p Θ = 100kpa 2 3 2 4 2 2 [ (Sn )][ (Fe )] [ (Sn )][ (Fe )] c /c c /c c /c c /c + + + + K = c Θ = 1 mol.l-1