正在加载图片...
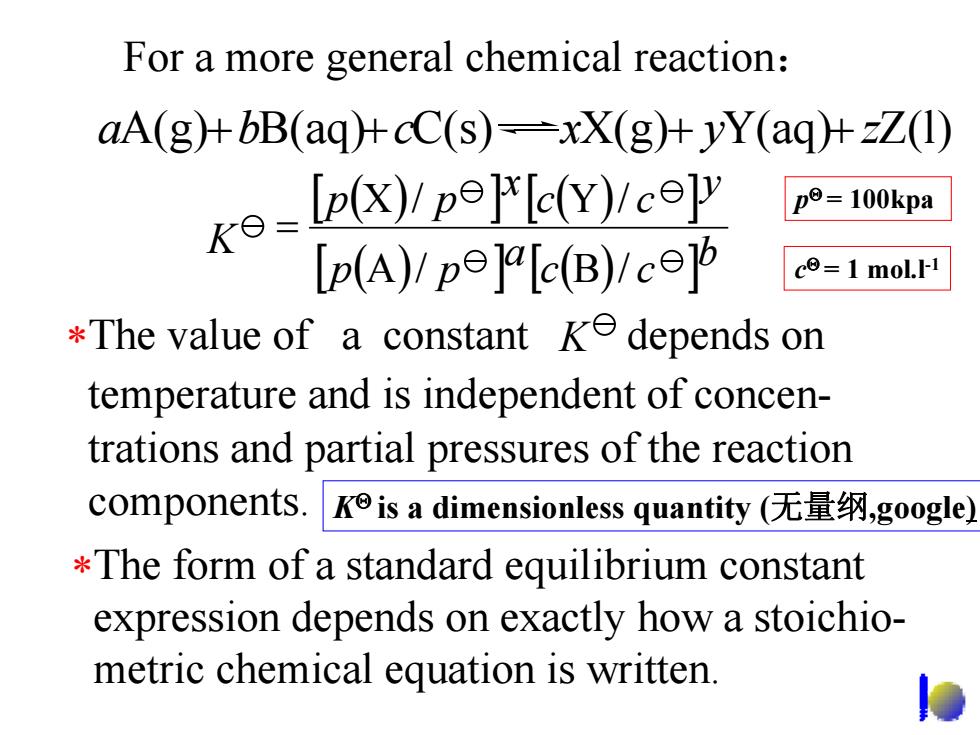
For a more general chemical reaction: aA(g)+bB(aq)+cC(s)-xX(g)+yY(aq)+zZ(l) exp)p p=100kpa [p(A)!pe]a[c(B)!c c=1 mol.I-1 *The value of a constant ke depends on temperature and is independent of concen- trations and partial pressures of the reaction components.Kis a dimensionless quantity(无量纲,google) The form of a standard equilibrium constant expression depends on exactly how a stoichio- metric chemical equation is written. leFor a more general chemical reaction : aA(g) + bB(aq)+ cC(s) xX(g) + yY(aq)+ zZ(l) K [ ( ) ] [ ( ) ] [ ] ( ) [ ] ( ) b c c a p p y c c x p p A / B / X / Y / = ∗The value of a constant depends on temperature and is independent of concentrations and partial pressures of the reaction components. K ∗The form of a standard equilibrium constant expression depends on exactly how a stoichiometric chemical equation is written. KΘ is a dimensionless quantity (无量纲,google) p Θ = 100kpa c Θ = 1 mol.l-1