正在加载图片...
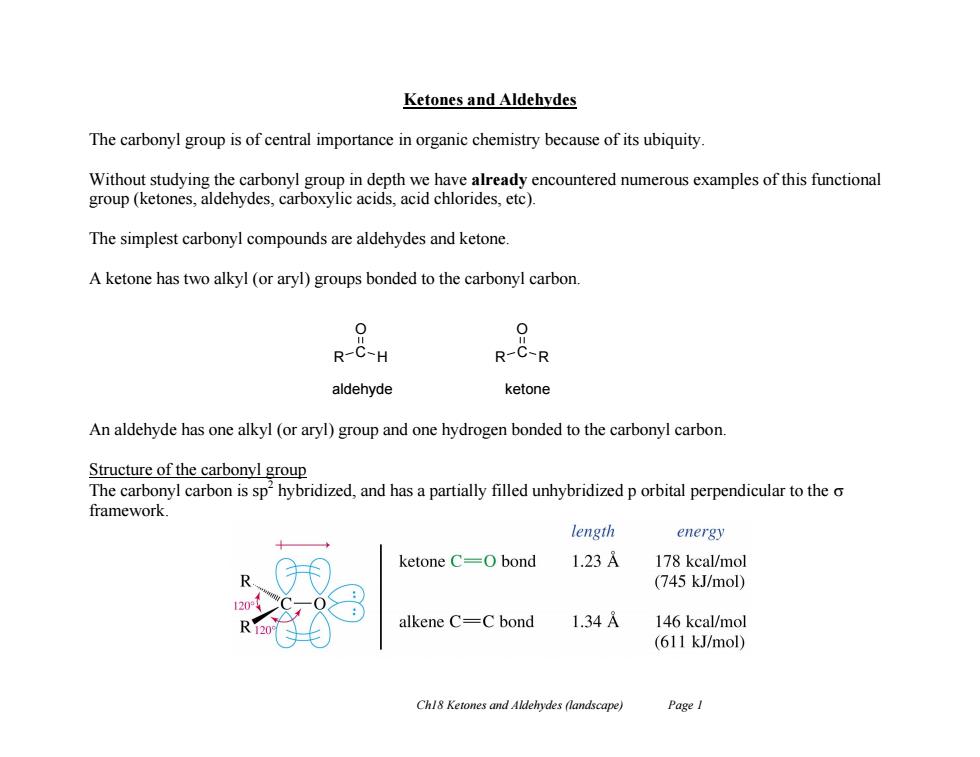
Ketones and Aldehydes The carbonyl group is of central importance in organic chemistry because of its ubiquity Without studying the carbonyl group in depth we have already encountered numerous examples of this functional group (ketones,aldehydes,carboxylic acids,acid chlorides.etc). The simplest carbonyl compounds are aldehydes and ketone. A ketone has two alkyl (or aryl)groups bonded to the carbonyl carbon. 0 R-C-H R-C-R aldehyde ketone An aldehyde has one alkyl (or aryl)group and one hydrogen bonded to the carbonyl carbon. Structure of the carbonyl group The carbonyl carbon is sp'hybridized,and has a partially filled unhybridized p orbital perpendicular to the o framework. length energy ketone C=0 bond 1.23A 178 kcal/mol (745 kJ/mol) alkene C=C bond 1.34 146 kcal/mol (611 kJ/mol) Chl8 Ketones and Aldehydes (landscape) Page I Ch18 Ketones and Aldehydes (landscape) Page 1 Ketones and Aldehydes The carbonyl group is of central importance in organic chemistry because of its ubiquity. Without studying the carbonyl group in depth we have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). The simplest carbonyl compounds are aldehydes and ketone. A ketone has two alkyl (or aryl) groups bonded to the carbonyl carbon. An aldehyde has one alkyl (or aryl) group and one hydrogen bonded to the carbonyl carbon. Structure of the carbonyl group The carbonyl carbon is sp2 hybridized, and has a partially filled unhybridized p orbital perpendicular to the framework. R C H O R C R O aldehyde ketone