正在加载图片...
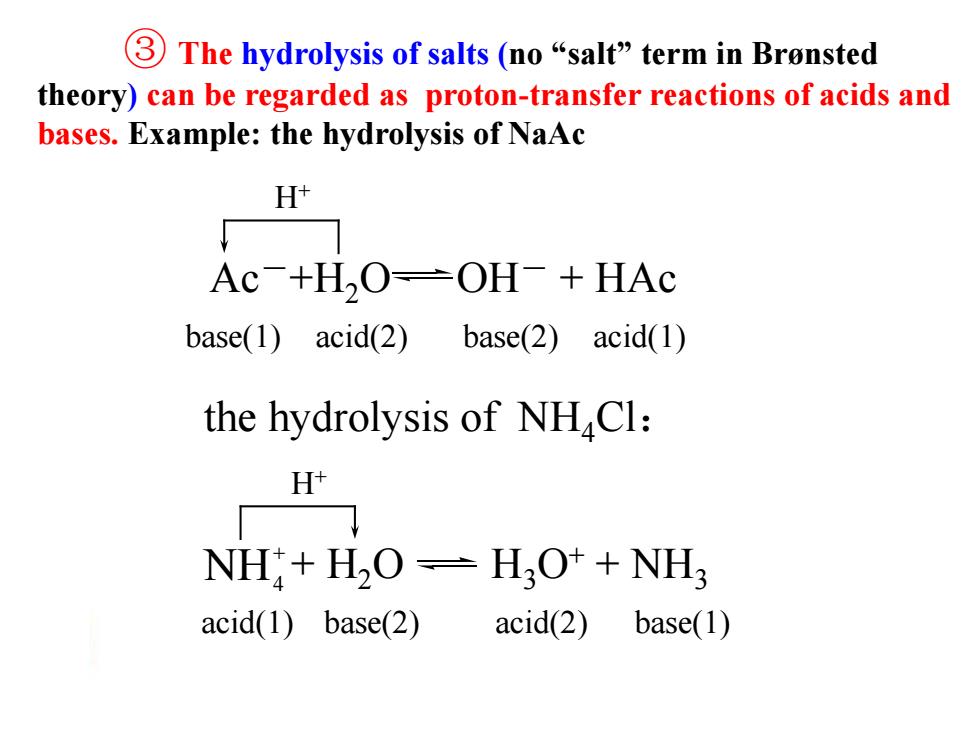
③The hydrolysis of salts(no“salt”term in Brensted theory)can be regarded as proton-transfer reactions of acids and bases.Example:the hydrolysis of NaAc H+ Ac-+H,O-OH+HAc base(1)acid(2)base(2 )acid(1) the hydrolysis of NHCl: H NH:+H2O=H2O++NH; acid(1)base(2) acid(2) base(1) ③ The hydrolysis of salts (no “salt” term in Brønsted theory) can be regarded as proton-transfer reactions of acids and bases. Example: the hydrolysis of NaAc base(1) acid(2) base(2) acid(1) H+ the hydrolysis of NH4Cl: acid(1) base(2) acid(2) base(1) H+ Ac-+H2O OH- + HAc + H2O H3O+ + NH3 + NH4