正在加载图片...
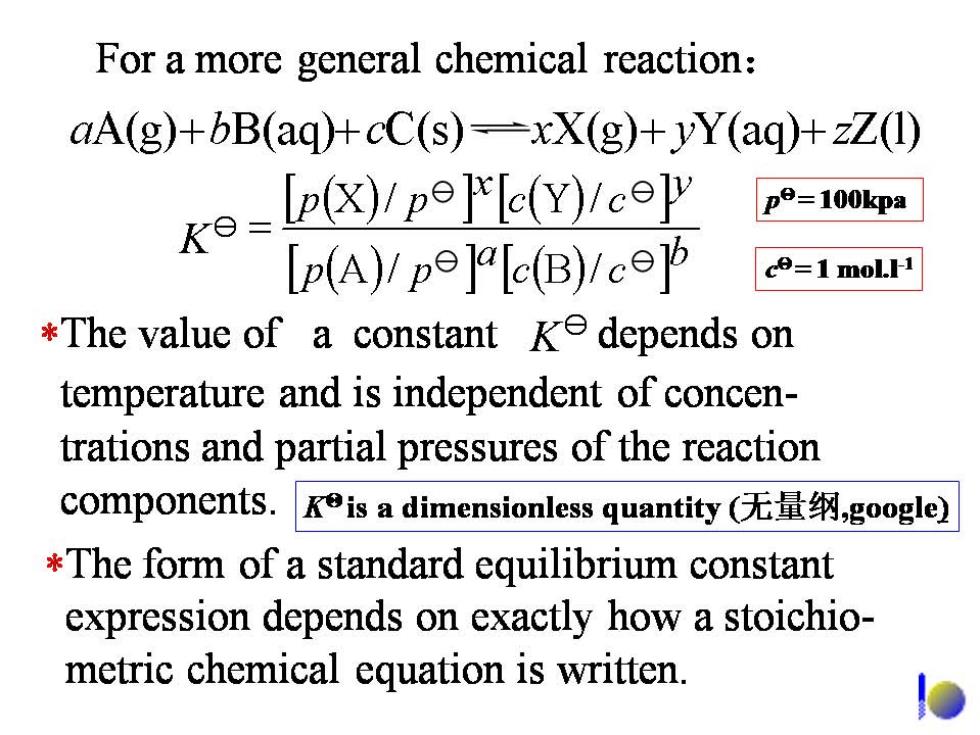
For a more general chemical reaction: aA(g)+bB(aq)+cC(s)=xX(g)+yY(aq)+zZ(1) Ke=pyp9]'eY/ceΨ pe=100kpa [p(A)/pe]r[c(B)1co的 co=1 molH *The value of a constant ke depends on temperature and is independent of concen- trations and partial pressures of the reaction components.Kis a dimensionless quantity(无量纲,google) *The form of a standard equilibrium constant expression depends on exactly how a stoichio- metric chemical equation is written