正在加载图片...
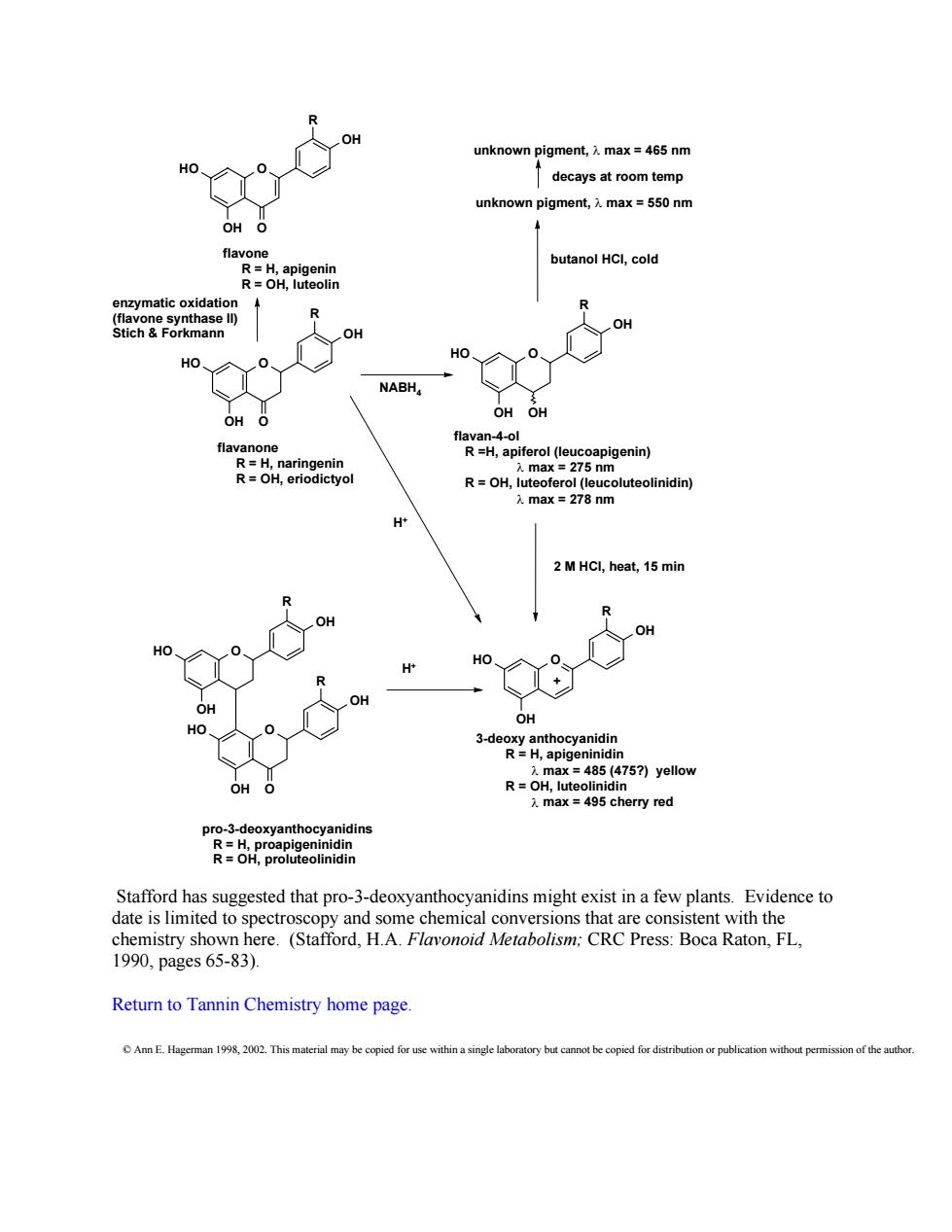
unknown pigment,max=465 nm decays at room temp unknown pigment,max=550 nm OH flavon butanol HCl,cold enaymatcoxidaa R R Stich&Forkmann OH OH HO、 NABH, OH O OH OH aaHpierole2g pigenin) R=OH,luteoferol(e oluteolinidin) max 278 nm H 2 MHCI,heat,15 min R OH HO H' HO、 3-de R.p R=OH 757)yellov 入max 95 cherry red pro-3-deoxyanthocyanidins Stafford has suggested that pro-3-deoxyanthocyanidins might exist in a few plants.Evidence to date is limited to spectroscopy and some chemical conversions that are consi tent with the chemistry shown here.(Stafford,H.A.Flavonoid Metabolism;CRC Press:Boca Raton,FL, 1990,pages65-83). Return to Tannin Chemistry home page. Ann E Hagerman 1998,2002.This material may be copied for use within a single b ned for d on of the author Stafford has suggested that pro-3-deoxyanthocyanidins might exist in a few plants. Evidence to date is limited to spectroscopy and some chemical conversions that are consistent with the chemistry shown here. (Stafford, H.A. Flavonoid Metabolism; CRC Press: Boca Raton, FL, 1990, pages 65-83). Return to Tannin Chemistry home page. © © Ann E. Hagerman 1998, 2002. This material may be copied for use within a single laboratory but cannot be copied for distribution or publication without permission of the author. HO O OH OH R O HO O OH OH R HO O OH OH R OH HO O OH OH R O HO O OH OH R HO O OH OH R O + enzymatic oxidation (flavone synthase II) Stich & Forkmann NABH4 H+ 2 M HCl, heat, 15 min butanol HCl, cold unknown pigment, λ max = 550 nm H+ flavone R = H, apigenin R = OH, luteolin unknown pigment, λ max = 465 nm decays at room temp 3-deoxy anthocyanidin R = H, apigeninidin λ max = 485 (475?) yellow R = OH, luteolinidin λ max = 495 cherry red flavan-4-ol R =H, apiferol (leucoapigenin) λ max = 275 nm R = OH, luteoferol (leucoluteolinidin) λ max = 278 nm flavanone R = H, naringenin R = OH, eriodictyol pro-3-deoxyanthocyanidins R = H, proapigeninidin R = OH, proluteolinidin