正在加载图片...
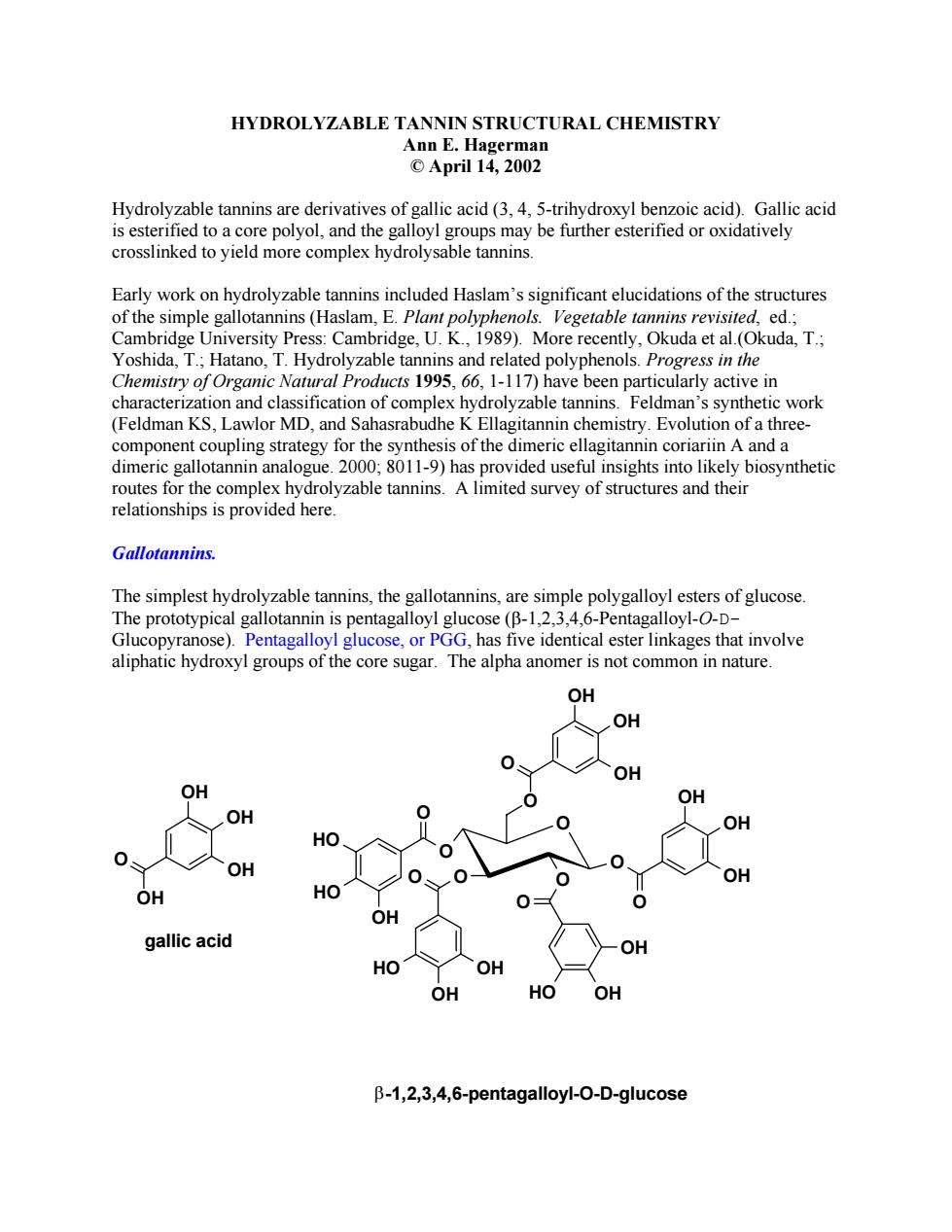
HYDROLYZABLE TANNIN STRUCTURAL CHEMISTRY Ann E.Hagerman ©April14,2002 Hydrolyzable tannins are derivatives of gallic acid(3,4,5-trihydroxyl benzoic acid).Gallic acid is esterified to a core polyol,and the galloyl groups may be further esterified or oxidatively crosslinked to yield more complex hydrolysable tannins. Early work on hydrolyzable tannins included Haslam's significant elucidations of the structures of the simple am,E.Plant enols.Vegetable idge. 1989).M ore recentl atan yza d polyphenols in the emistry of Organi .1-117)have been particula y active in racteri n an fica drolyzable tar ns. etic work (Feldman KS.L of comple awlor MD,and Sahasrab ekeminchemaEotuimiae component coupling strategy for the synthesis of the dimeric ellagitannin coriariin A and a dimeric gallotannin analogue.2000;8011-9)has provided useful insights into likely biosynthetic routes for the complex hydrolyzable tannins.A limited survey of structures and their relationships is provided here. Gallotannins. The simplest hydrolyzable tannins,the gallotannins,are simple polygalloyl esters of glucose The prototypical gallotannin is pentagalloyl glucose(B-1,2,3,4,6-Pentagalloyl-O-D- Gluc se).Pentagalloyl glucose,or PGG.has five identical ester linkages that involve ugar.The alpha anomer is not com OH OH OH OH OH OH OH OH gallic acid O HO OH HO OH B-1,2,3,4,6-pentagalloyl-O-D-glucoseHYDROLYZABLE TANNIN STRUCTURAL CHEMISTRY Ann E. Hagerman © April 14, 2002 Hydrolyzable tannins are derivatives of gallic acid (3, 4, 5-trihydroxyl benzoic acid). Gallic acid is esterified to a core polyol, and the galloyl groups may be further esterified or oxidatively crosslinked to yield more complex hydrolysable tannins. Early work on hydrolyzable tannins included Haslam’s significant elucidations of the structures of the simple gallotannins (Haslam, E. Plant polyphenols. Vegetable tannins revisited, ed.; Cambridge University Press: Cambridge, U. K., 1989). More recently, Okuda et al.(Okuda, T.; Yoshida, T.; Hatano, T. Hydrolyzable tannins and related polyphenols. Progress in the Chemistry of Organic Natural Products 1995, 66, 1-117) have been particularly active in characterization and classification of complex hydrolyzable tannins. Feldman’s synthetic work (Feldman KS, Lawlor MD, and Sahasrabudhe K Ellagitannin chemistry. Evolution of a threecomponent coupling strategy for the synthesis of the dimeric ellagitannin coriariin A and a dimeric gallotannin analogue. 2000; 8011-9) has provided useful insights into likely biosynthetic routes for the complex hydrolyzable tannins. A limited survey of structures and their relationships is provided here. Gallotannins. The simplest hydrolyzable tannins, the gallotannins, are simple polygalloyl esters of glucose. The prototypical gallotannin is pentagalloyl glucose (β-1,2,3,4,6-Pentagalloyl-O-DGlucopyranose). Pentagalloyl glucose, or PGG, has five identical ester linkages that involve aliphatic hydroxyl groups of the core sugar. The alpha anomer is not common in nature. O OH HO OH O O OH OH HO O O OH HO HO O O OH OH OH O O OH OH OH O O OH OH OH OH O β-1,2,3,4,6-pentagalloyl-O-D-glucose gallic acid