正在加载图片...
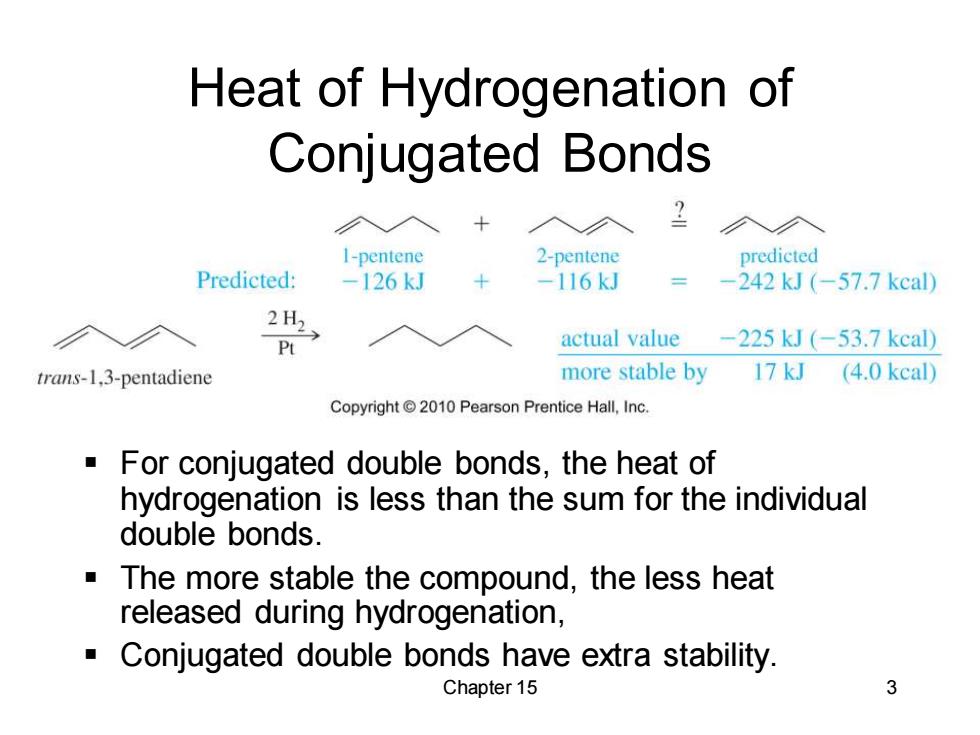
Heat of Hydrogenation of Conjugated Bonds I-pentene 2-pentene predicted Predicted: -126kJ -116kJ -242kJ(-57.7kcal) 2H2 actual value -225kJ(-53.7kcal) trans-1,3-pentadiene more stable by 17 kJ (4.0 kcal) Copyright 2010 Pearson Prentice Hall,Inc. For conjugated double bonds,the heat of hydrogenation is less than the sum for the individual double bonds. The more stable the compound,the less heat released during hydrogenation, Conjugated double bonds have extra stability. Chapter 15 3Chapter 15 3 Heat of Hydrogenation of Conjugated Bonds ▪ For conjugated double bonds, the heat of hydrogenation is less than the sum for the individual double bonds. ▪ The more stable the compound, the less heat released during hydrogenation, ▪ Conjugated double bonds have extra stability