正在加载图片...
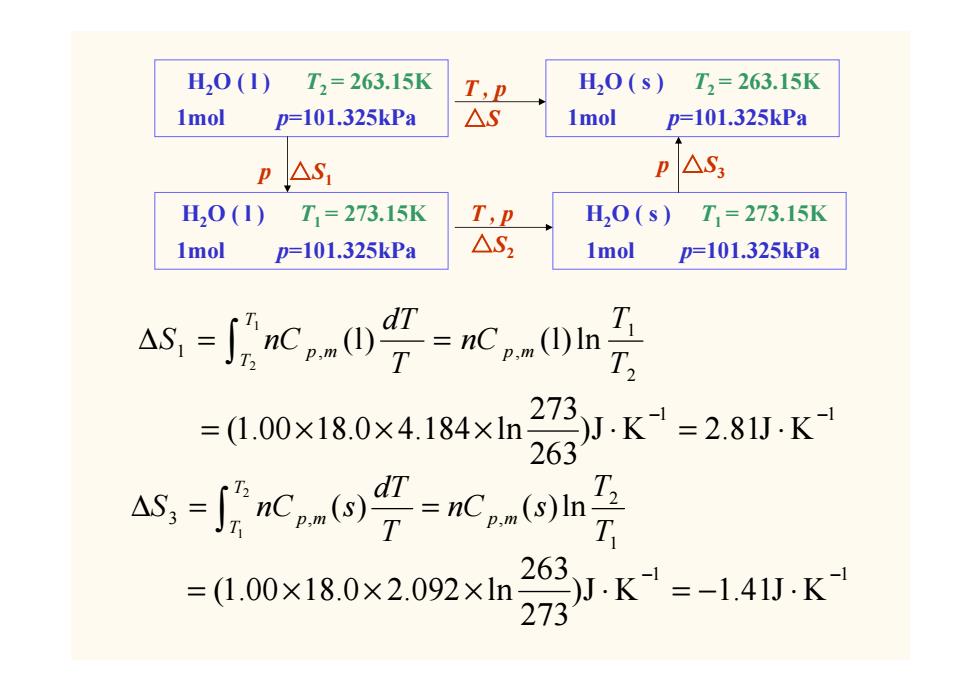
H,0(1) T2=263.15K T,P H,0(s)T2=263.15K 1mol p=101.325kPa △S 1mol p=101.325kPa P AS p△S3 H20(1) T1=273.15K T,P H0(s) T1=273.15K 1mol p=101.325kPa △S2 1mol p=101.325kPa As,-Jcg-c0n 273 =(1.00×18.0×4.184×ln JK1=2.81JK 263 =ao9=m9 3 T 263 =(1.00×18.0×2.092×ln )J.K=-1.41JK 273 H2O ( l ) T2 = 263.15K 1mol p=101.325kPa H2O ( s ) T2 = 263.15K 1mol p=101.325kPa H2O ( l ) T1 = 273.15K 1mol p=101.325kPa H2O ( s ) T1 = 273.15K 1mol p=101.325kPa T , p △S T , p △S2 p △S1 p △S3 ∫ ∆ = = 12 21 1 , , (l) (l)ln TT p m p m TT nC TdT S nC 1 1 )J K 2.81J K 263 273 (1.00 18.0 4.184 ln − − = × × × ⋅ = ⋅ 1 2 3 , , ( ) ( )ln 2 1 T T nC s T dT S nC s p m TT ∆ = p m = ∫ 1 1 )J K 1.41J K 273 263 (1.00 18.0 2.092 ln − − = × × × ⋅ = − ⋅