正在加载图片...
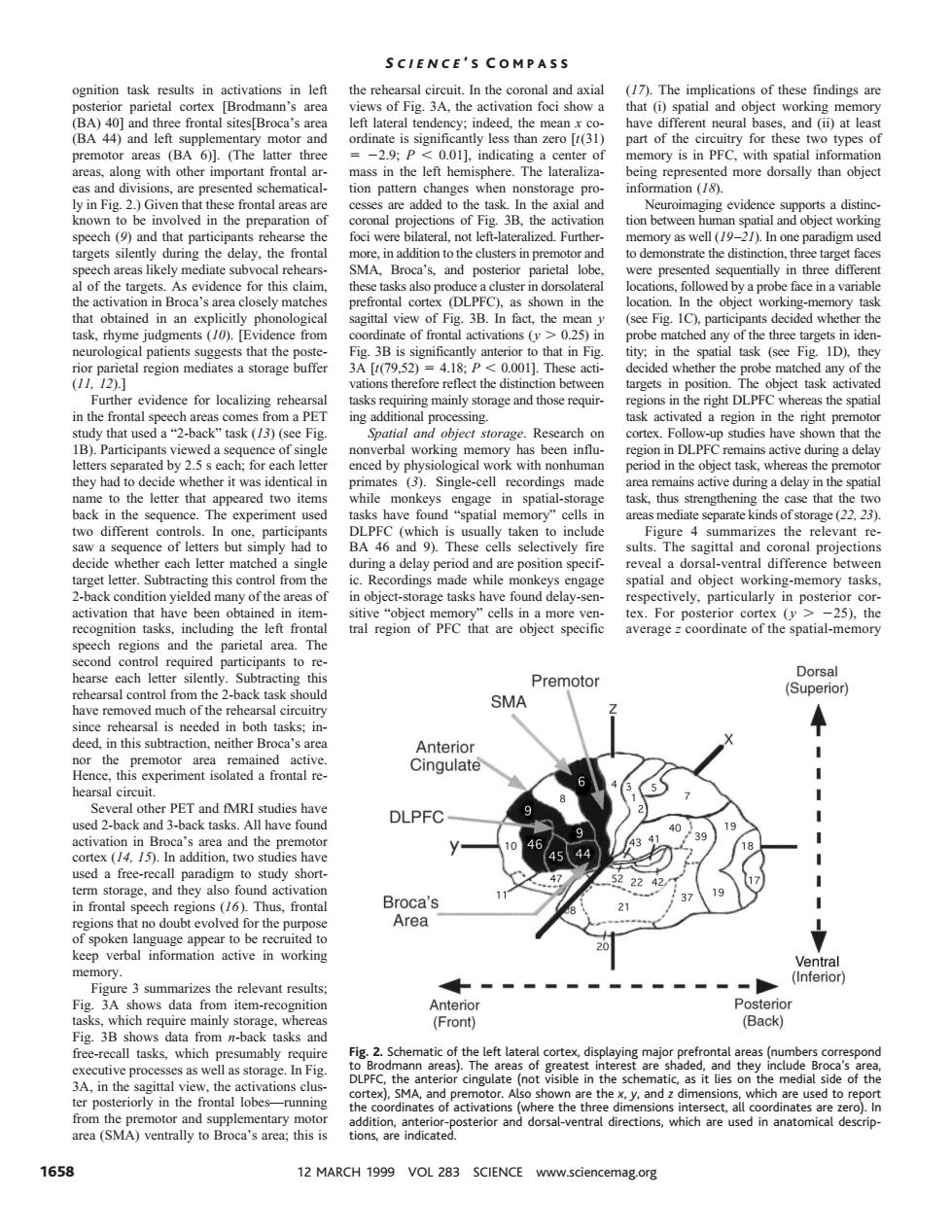
SCIENCE'S COMPASS al eireuit in the c of thes s of Fig.3A.the (BA 44) 201 nory is in PF ed sch matical on pat information (18). distin o be the atio etioe tior tion between】 n sp ct workin inpremotor n on,three target face lof the evid and Broca's d in ica of see Fig. rhyme judgments 10. ce from of fr ons 0.25 ide tal region mediates a storage buffer [t79.52 418 0.001].Thes of t evidence for localizin asks r ring mainly s and tho n t that used a?-back region the righ ce of: has been influ gion n DLPF ing a dela y had identical in recordings ve during ack in the seau use rea 2223 but s ult The sagittal and ask orage tasks vely,particularly tasks averagecoordinate of the spatial-memory Premotor Dorsal o from the task SMA (Supe ded in both in this subtraction.neit Cingulate and MRI studies have have found DLPFC 44 47 224 an Broca's tn for the pur Area (n ws data from n-back and Fig.2.Sch cof the left isible the s of th ations ci sterior and dorsal-ventral directions which are used in anatomical descrip 1658 12 MARCH 1999 VOL 283 SCIENCE www.sciencemag.org ognition task results in activations in left posterior parietal cortex [Brodmann’s area (BA) 40] and three frontal sites[Broca’s area (BA 44) and left supplementary motor and premotor areas (BA 6)]. (The latter three areas, along with other important frontal areas and divisions, are presented schematically in Fig. 2.) Given that these frontal areas are known to be involved in the preparation of speech (9) and that participants rehearse the targets silently during the delay, the frontal speech areas likely mediate subvocal rehearsal of the targets. As evidence for this claim, the activation in Broca’s area closely matches that obtained in an explicitly phonological task, rhyme judgments (10). [Evidence from neurological patients suggests that the posterior parietal region mediates a storage buffer (11, 12).] Further evidence for localizing rehearsal in the frontal speech areas comes from a PET study that used a “2-back” task (13) (see Fig. 1B). Participants viewed a sequence of single letters separated by 2.5 s each; for each letter they had to decide whether it was identical in name to the letter that appeared two items back in the sequence. The experiment used two different controls. In one, participants saw a sequence of letters but simply had to decide whether each letter matched a single target letter. Subtracting this control from the 2-back condition yielded many of the areas of activation that have been obtained in itemrecognition tasks, including the left frontal speech regions and the parietal area. The second control required participants to rehearse each letter silently. Subtracting this rehearsal control from the 2-back task should have removed much of the rehearsal circuitry since rehearsal is needed in both tasks; indeed, in this subtraction, neither Broca’s area nor the premotor area remained active. Hence, this experiment isolated a frontal rehearsal circuit. Several other PET and fMRI studies have used 2-back and 3-back tasks. All have found activation in Broca’s area and the premotor cortex (14, 15). In addition, two studies have used a free-recall paradigm to study shortterm storage, and they also found activation in frontal speech regions (16). Thus, frontal regions that no doubt evolved for the purpose of spoken language appear to be recruited to keep verbal information active in working memory. Figure 3 summarizes the relevant results; Fig. 3A shows data from item-recognition tasks, which require mainly storage, whereas Fig. 3B shows data from n-back tasks and free-recall tasks, which presumably require executive processes as well as storage. In Fig. 3A, in the sagittal view, the activations cluster posteriorly in the frontal lobes—running from the premotor and supplementary motor area (SMA) ventrally to Broca’s area; this is the rehearsal circuit. In the coronal and axial views of Fig. 3A, the activation foci show a left lateral tendency; indeed, the mean x coordinate is significantly less than zero [t(31) 5 22.9; P , 0.01], indicating a center of mass in the left hemisphere. The lateralization pattern changes when nonstorage processes are added to the task. In the axial and coronal projections of Fig. 3B, the activation foci were bilateral, not left-lateralized. Furthermore, in addition to the clusters in premotor and SMA, Broca’s, and posterior parietal lobe, these tasks also produce a cluster in dorsolateral prefrontal cortex (DLPFC), as shown in the sagittal view of Fig. 3B. In fact, the mean y coordinate of frontal activations (y . 0.25) in Fig. 3B is significantly anterior to that in Fig. 3A [t(79,52) 5 4.18; P , 0.001]. These activations therefore reflect the distinction between tasks requiring mainly storage and those requiring additional processing. Spatial and object storage. Research on nonverbal working memory has been influenced by physiological work with nonhuman primates (3). Single-cell recordings made while monkeys engage in spatial-storage tasks have found “spatial memory” cells in DLPFC (which is usually taken to include BA 46 and 9). These cells selectively fire during a delay period and are position specific. Recordings made while monkeys engage in object-storage tasks have found delay-sensitive “object memory” cells in a more ventral region of PFC that are object specific (17). The implications of these findings are that (i) spatial and object working memory have different neural bases, and (ii) at least part of the circuitry for these two types of memory is in PFC, with spatial information being represented more dorsally than object information (18). Neuroimaging evidence supports a distinction between human spatial and object working memory as well (19–21). In one paradigm used to demonstrate the distinction, three target faces were presented sequentially in three different locations, followed by a probe face in a variable location. In the object working-memory task (see Fig. 1C), participants decided whether the probe matched any of the three targets in identity; in the spatial task (see Fig. 1D), they decided whether the probe matched any of the targets in position. The object task activated regions in the right DLPFC whereas the spatial task activated a region in the right premotor cortex. Follow-up studies have shown that the region in DLPFC remains active during a delay period in the object task, whereas the premotor area remains active during a delay in the spatial task, thus strengthening the case that the two areas mediate separate kinds of storage (22, 23). Figure 4 summarizes the relevant results. The sagittal and coronal projections reveal a dorsal-ventral difference between spatial and object working-memory tasks, respectively, particularly in posterior cortex. For posterior cortex ( y . 225), the average z coordinate of the spatial-memory Fig. 2. Schematic of the left lateral cortex, displaying major prefrontal areas (numbers correspond to Brodmann areas). The areas of greatest interest are shaded, and they include Broca’s area, DLPFC, the anterior cingulate (not visible in the schematic, as it lies on the medial side of the cortex), SMA, and premotor. Also shown are the x, y, and z dimensions, which are used to report the coordinates of activations (where the three dimensions intersect, all coordinates are zero). In addition, anterior-posterior and dorsal-ventral directions, which are used in anatomical descriptions, are indicated. S CIENCE ’ S C OMPASS 1658 12 MARCH 1999 VOL 283 SCIENCE www.sciencemag.org