正在加载图片...
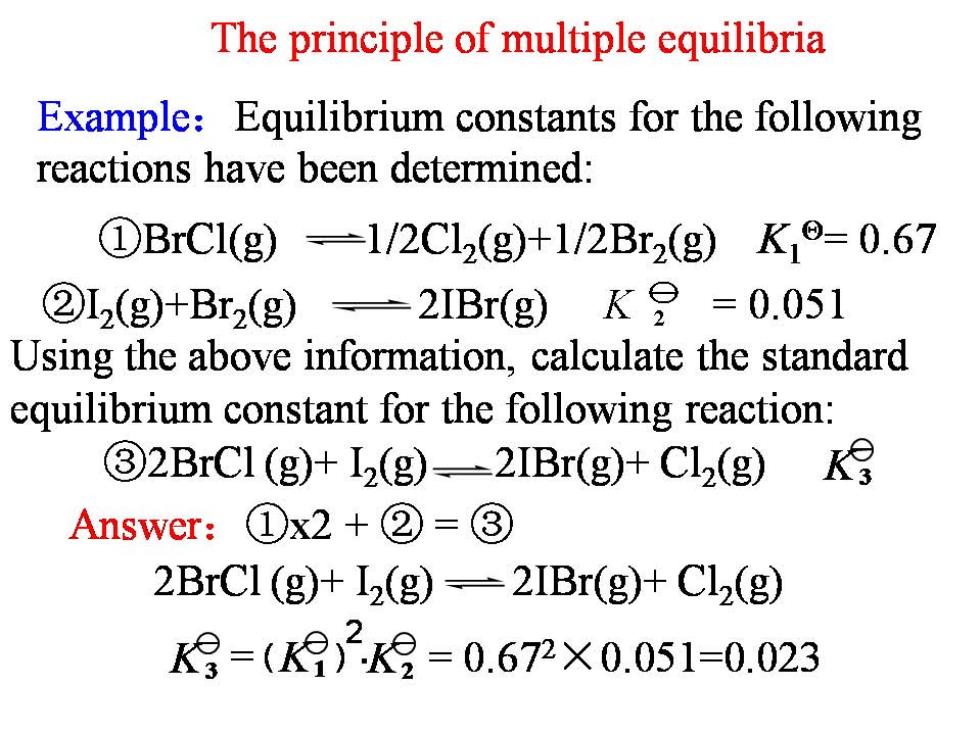
The principle of multiple equilibria Example:Equilibrium constants for the following reactions have been determined: ①BrC1(g)=1/2Cl2(g+1/2Br2(gK19=0.67 ②L2(g)+Br2(g)=2IBr(g)K9=0.051 Using the above information,calculate the standard equilibrium constant for the following reaction: 32BrCl (g)+I2(g)-2IBr(g)+Cl2(g) Answer:①x2+②=③ 2BrCl(g)+I2(g)=2IBr(g)+Cl2(g) K9=()2=0.672×0.051=0.023