正在加载图片...
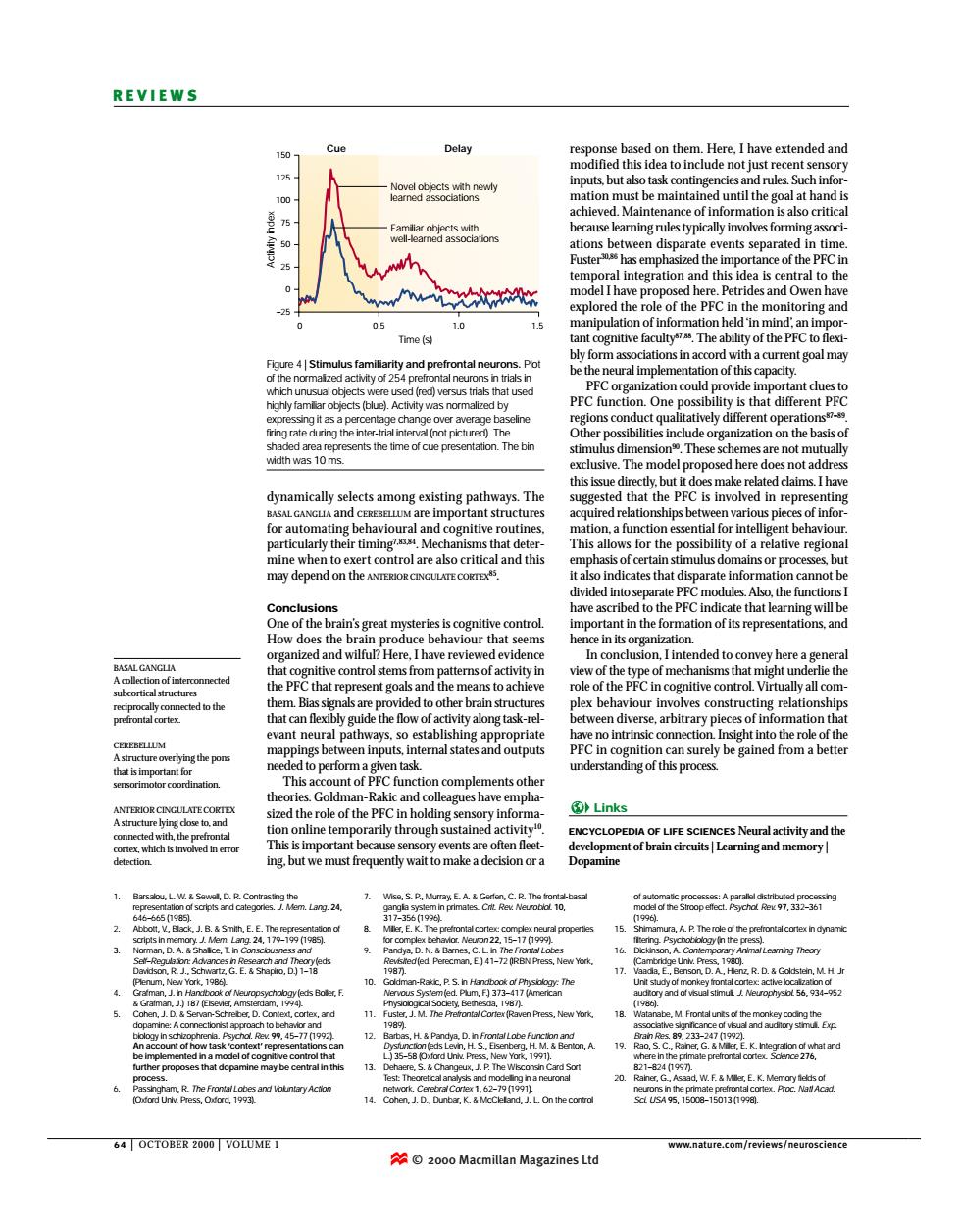
REVIEWS Delay ebasedon them.Here.Ihave ask of inf ents s in ti e of nteeration nd this idea is d the role of the PEC Tme 1.0 PEC O orga PE ge che (no with was 10ms. These ed claims.I hav ally sela tstructure ips betv of info rly their timing for the may depend on the al indicates that isparate informa ave as the PF ing will h How e beh iour t itive control sten pe ot r hen led to other be our inv Ives constru t neural pathways no intrinsi le of t pu r9 Gold -Rak olptmentk Links arily thr ENCYCLOPEDIA OF LFE SCIENCES Neural activity and the gpiopecmtofbraindiaislLkanmtgandmcamo 56.934- ess.New Yor 1 ncin and 35-5 dopamine may b d,J.L.On the conerol 54 OCTOBER 2000 VOLUME I 2000 Macmillan Magazines Lto 64 | OCTOBER 2000 | VOLUME 1 www.nature.com/reviews/neuroscience REVIEWS response based on them. Here, I have extended and modified this idea to include not just recent sensory inputs, but also task contingencies and rules. Such information must be maintained until the goal at hand is achieved. Maintenance of information is also critical because learning rules typically involves forming associations between disparate events separated in time. Fuster30,86 has emphasized the importance of the PFC in temporal integration and this idea is central to the model I have proposed here. Petrides and Owen have explored the role of the PFC in the monitoring and manipulation of information held ‘in mind’, an important cognitive faculty87,88. The ability of the PFC to flexibly form associations in accord with a current goal may be the neural implementation of this capacity. PFC organization could provide important clues to PFC function. One possibility is that different PFC regions conduct qualitatively different operations87–89. Other possibilities include organization on the basis of stimulus dimension90. These schemes are not mutually exclusive. The model proposed here does not address this issue directly, but it does make related claims. I have suggested that the PFC is involved in representing acquired relationships between various pieces of information, a function essential for intelligent behaviour. This allows for the possibility of a relative regional emphasis of certain stimulus domains or processes, but it also indicates that disparate information cannot be divided into separate PFC modules. Also, the functions I have ascribed to the PFC indicate that learning will be important in the formation of its representations, and hence in its organization. In conclusion, I intended to convey here a general view of the type of mechanisms that might underlie the role of the PFC in cognitive control. Virtually all complex behaviour involves constructing relationships between diverse, arbitrary pieces of information that have no intrinsic connection. Insight into the role of the PFC in cognition can surely be gained from a better understanding of this process. dynamically selects among existing pathways. The BASAL GANGLIA and CEREBELLUM are important structures for automating behavioural and cognitive routines, particularly their timing7,83,84. Mechanisms that determine when to exert control are also critical and this may depend on the ANTERIOR CINGULATE CORTEX85. Conclusions One of the brain’s great mysteries is cognitive control. How does the brain produce behaviour that seems organized and wilful? Here, I have reviewed evidence that cognitive control stems from patterns of activity in the PFC that represent goals and the means to achieve them. Bias signals are provided to other brain structures that can flexibly guide the flow of activity along task-relevant neural pathways, so establishing appropriate mappings between inputs, internal states and outputs needed to perform a given task. This account of PFC function complements other theories. Goldman-Rakic and colleagues have emphasized the role of the PFC in holding sensory information online temporarily through sustained activity10. This is important because sensory events are often fleeting, but we must frequently wait to make a decision or a BASAL GANGLIA A collection of interconnected subcortical structures reciprocally connected to the prefrontal cortex. CEREBELLUM A structure overlying the pons that is important for sensorimotor coordination. ANTERIOR CINGULATE CORTEX A structure lying close to, and connected with, the prefrontal cortex, which is involved in error detection. 150 125 100 75 50 25 0 –25 0 0.5 Time (s) Cue Delay Novel objects with newly learned associations Familiar objects with well-learned associations Activity index 1.0 1.5 Figure 4 | Stimulus familiarity and prefrontal neurons. Plot of the normalized activity of 254 prefrontal neurons in trials in which unusual objects were used (red) versus trials that used highly familiar objects (blue). Activity was normalized by expressing it as a percentage change over average baseline firing rate during the inter-trial interval (not pictured). The shaded area represents the time of cue presentation. The bin width was 10 ms. Links ENCYCLOPEDIA OF LIFE SCIENCES Neural activity and the development of brain circuits | Learning and memory | Dopamine 1. Barsalou, L. W. & Sewell, D. R. Contrasting the representation of scripts and categories. J. Mem. Lang. 24, 646–665 (1985). 2. Abbott, V., Black, J. B. & Smith, E. E. The representation of scripts in memory. J. Mem. Lang. 24, 179–199 (1985). 3. Norman, D. A. & Shallice, T. in Consciousness and Self–Regulation: Advances in Research and Theory (eds Davidson, R. J., Schwartz, G. E. & Shapiro, D.) 1–18 (Plenum, New York, 1986). 4. Grafman, J. in Handbook of Neuropsychology (eds Boller, F. & Grafman, J.) 187 (Elsevier, Amsterdam, 1994). 5. Cohen, J. D. & Servan-Schreiber, D. Context, cortex, and dopamine: A connectionist approach to behavior and biology in schizophrenia. Psychol. Rev. 99, 45–77 (1992). An account of how task ‘context’ representations can be implemented in a model of cognitive control that further proposes that dopamine may be central in this process. 6. Passingham, R. The Frontal Lobes and Voluntary Action (Oxford Univ. Press, Oxford, 1993). 7. Wise, S. P., Murray, E. A. & Gerfen, C. R. The frontal-basal ganglia system in primates. Crit. Rev. Neurobiol. 10, 317–356 (1996). 8. Miller, E. K. The prefrontal cortex: complex neural properties for complex behavior. Neuron 22, 15–17 (1999). 9. Pandya, D. N. & Barnes, C. L. in The Frontal Lobes Revisited (ed. Perecman, E.) 41–72 (IRBN Press, New York, 1987). 10. Goldman-Rakic, P. S. in Handbook of Physiology: The Nervous System (ed. Plum, F.) 373–417 (American Physiological Society, Bethesda, 1987). 11. Fuster, J. M. The Prefrontal Cortex (Raven Press, New York, 1989). 12. Barbas, H. & Pandya, D. in Frontal Lobe Function and Dysfunction (eds Levin, H. S., Eisenberg, H. M. & Benton, A. L.) 35–58 (Oxford Univ. Press, New York, 1991). 13. Dehaere, S. & Changeux, J. P. The Wisconsin Card Sort Test: Theoretical analysis and modelling in a neuronal network. Cerebral Cortex 1, 62–79 (1991). 14. Cohen, J. D., Dunbar, K. & McClelland, J. L. On the control of automatic processes: A parallel distributed processing model of the Stroop effect. Psychol. Rev. 97, 332–361 (1996). 15. Shimamura, A. P. The role of the prefrontal cortex in dynamic filtering. Psychobiology (in the press). 16. Dickinson, A. Contemporary Animal Learning Theory (Cambridge Univ. Press, 1980). 17. Vaadia, E., Benson, D. A., Hienz, R. D. & Goldstein, M. H. Jr Unit study of monkey frontal cortex: active localization of auditory and of visual stimuli. J. Neurophysiol. 56, 934–952 (1986). 18. Watanabe, M. Frontal units of the monkey coding the associative significance of visual and auditory stimuli. Exp. Brain Res. 89, 233–247 (1992). 19. Rao, S. C., Rainer, G. & Miller, E. K. Integration of what and where in the primate prefrontal cortex. Science 276, 821–824 (1997). 20. Rainer, G., Asaad, W. F. & Miller, E. K. Memory fields of neurons in the primate prefrontal cortex. Proc. Natl Acad. Sci. USA 95, 15008–15013 (1998). © 2000 Macmillan Magazines Ltd