正在加载图片...
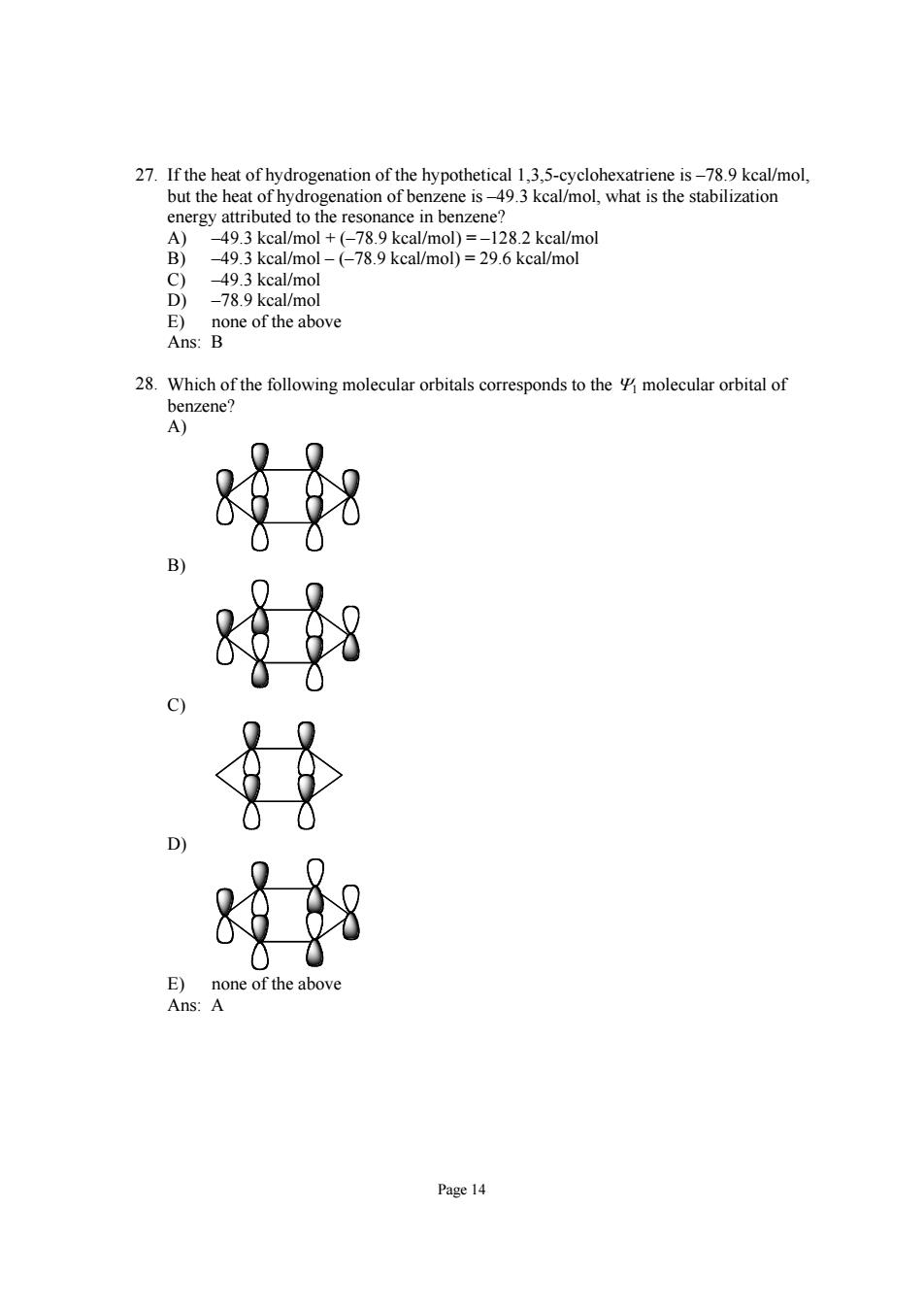
27.If the heat of hydrogenation of the hypothetical 1.3.5-cyclohexatriene is-78.9 kcal/mol. 一on6 kcal/mol kcal/moD)=296 kcalmol 49.3 kcal/mol D)-78.9 kcal/mol E)none of the above Ans:B 28.Which of the following molecular orbitals coresponds to nzene A 对粉 Page 14Page 14 27. If the heat of hydrogenation of the hypothetical 1,3,5-cyclohexatriene is –78.9 kcal/mol, but the heat of hydrogenation of benzene is –49.3 kcal/mol, what is the stabilization energy attributed to the resonance in benzene? A) –49.3 kcal/mol + (–78.9 kcal/mol) = –128.2 kcal/mol B) –49.3 kcal/mol – (–78.9 kcal/mol) = 29.6 kcal/mol C) –49.3 kcal/mol D) –78.9 kcal/mol E) none of the above Ans: B 28. Which of the following molecular orbitals corresponds to the Ψ1 molecular orbital of benzene? A) B) C) D) E) none of the above Ans: A