正在加载图片...
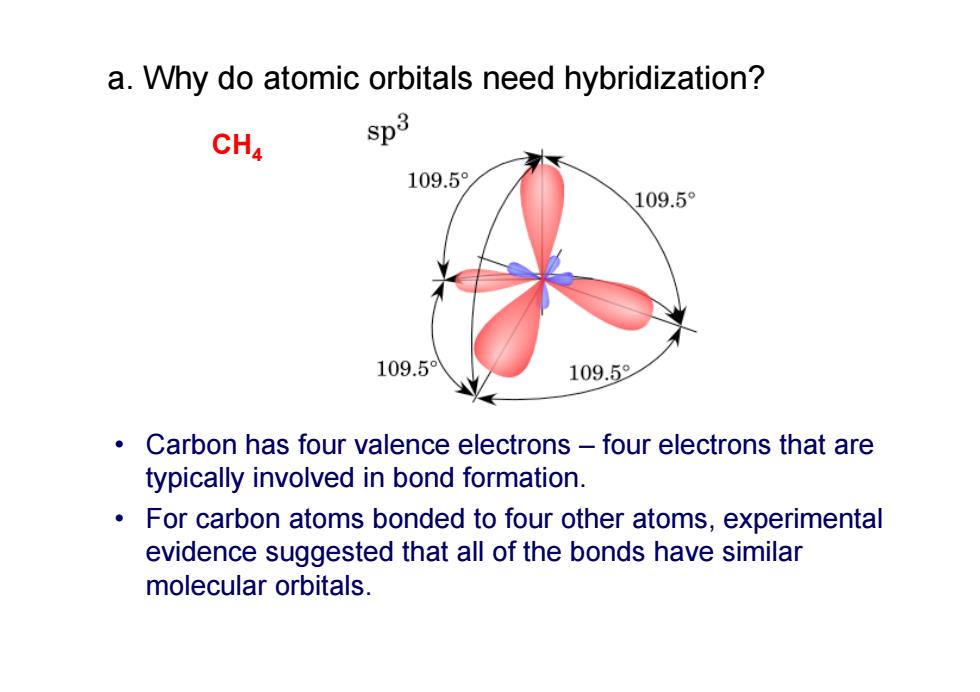
a.Why do atomic orbitals need hybridization? CH4 $p3 109.5 109.5° 109.5 109.5 Carbon has four valence electrons-four electrons that are typically involved in bond formation. For carbon atoms bonded to four other atoms,experimental evidence suggested that all of the bonds have similar molecular orbitals.• Carbon has four valence electrons – four electrons that are typically involved in bond formation. • For carbon atoms bonded to four other atoms, experimental evidence suggested that all of the bonds have similar molecular orbitals. CH 4 a. Why do atomic orbitals need hybridization?