正在加载图片...
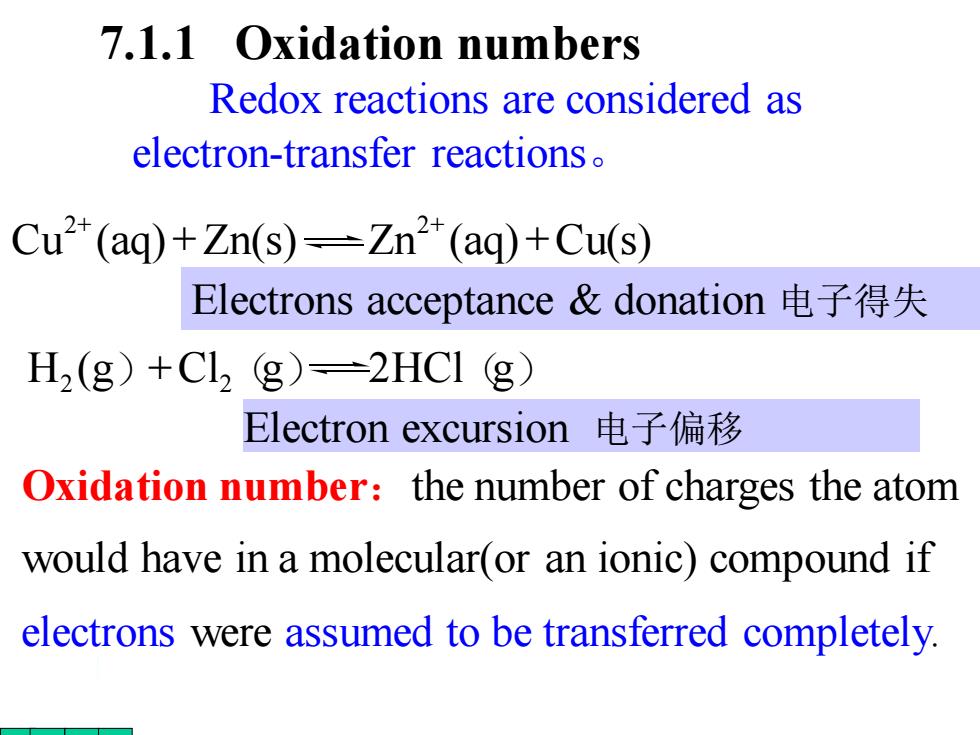
7.1.1 Oxidation numbers Redox reactions are considered as electron-transfer reactions Cu2(aq)+Zn(s)=Zn(aq)+Cu(s) Electrons acceptance&donation电子得失 H2(g)+Cl2 g)=2HCI (g) Electron excursion电子偏移 Oxidation number:the number of charges the atom would have in a molecular(or an ionic)compound if electrons were assumed to be transferred completely. 7.1.1 Oxidation numbers Oxidation number:the number of charges the atom would have in a molecular(or an ionic) compound if electrons were assumed to be transferred completely. Redox reactions are considered as electron-transfer reactions。 Cu (aq) Zn(s) Zn (aq) Cu(s) 2 2 + + + + Electron excursion 电子偏移 H2 (g)+Cl2(g) 2HCl(g) Electrons acceptance & donation 电子得失