正在加载图片...
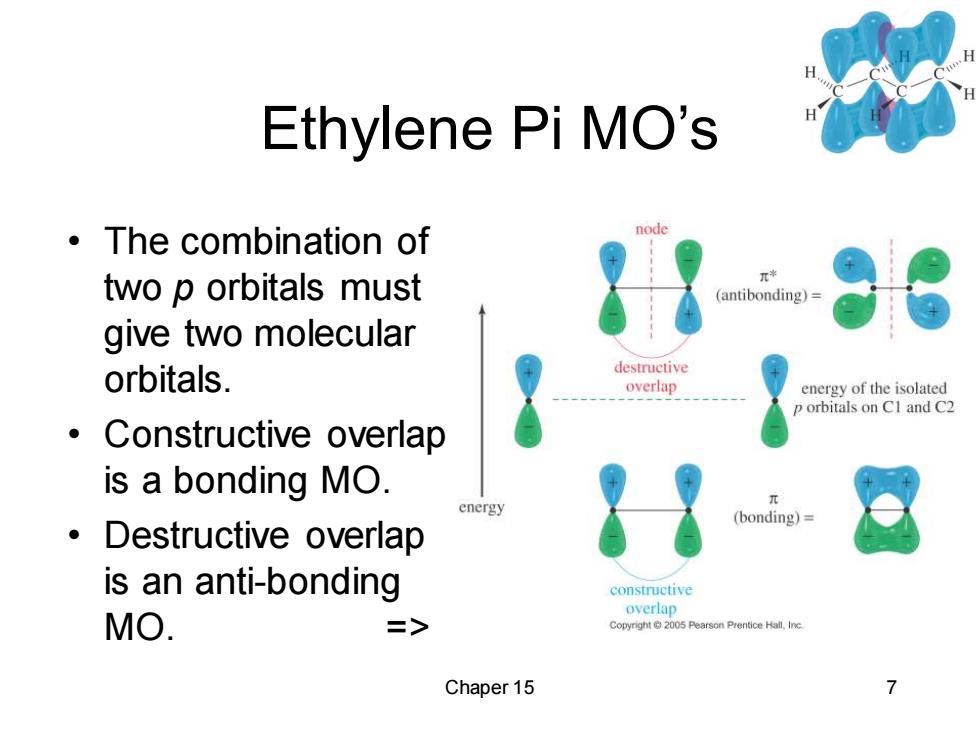
Ethylene Pi MO's ·The combination of node two p orbitals must π* (antibonding)= give two molecular orbitals. destructive overlap energy of the isolated p orbitals on CI and C2 ● Constructive overlap is a bonding MO. energy (bonding)= 。Destructive overlap is an anti-bonding constructive overlap MO. => Copyright2005 Pearson Prentice Hall.Inc Chaper 15 Chaper 15 7 Ethylene Pi MO’s • The combination of two p orbitals must give two molecular orbitals. • Constructive overlap is a bonding MO. • Destructive overlap is an anti-bonding MO. =>