正在加载图片...
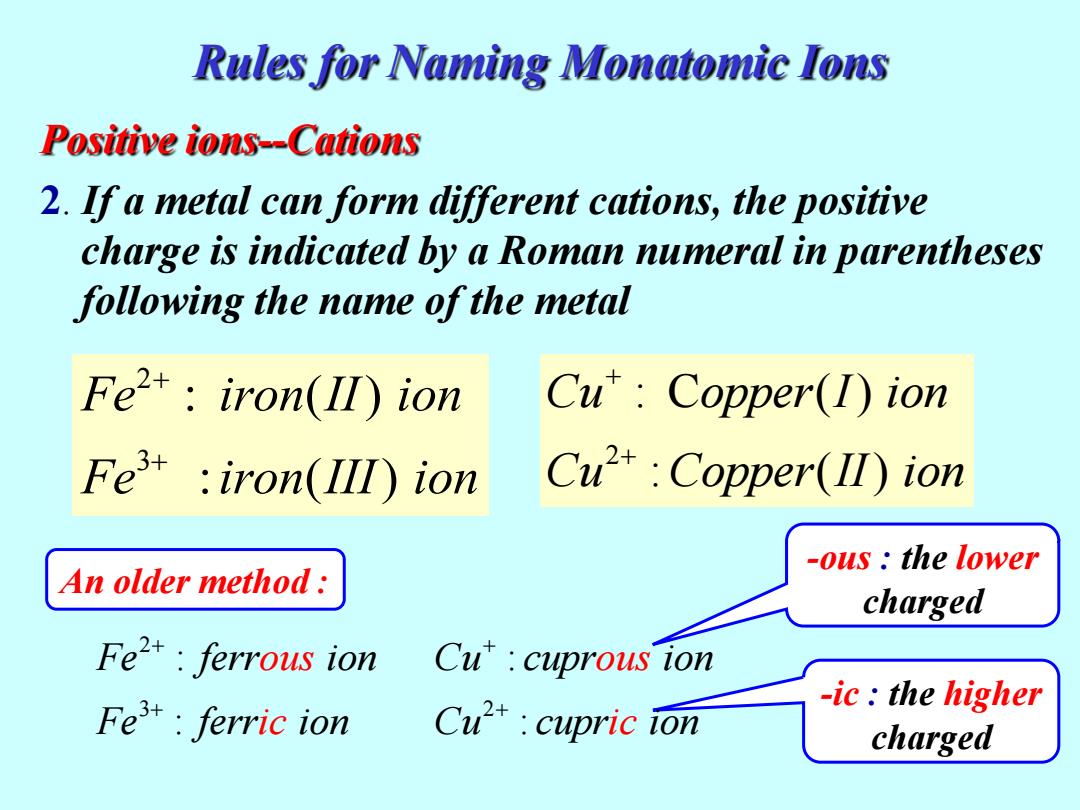
Rules for Naming Monatomic Ions Positive ions--Cations 2.If a metal can form different cations,the positive charge is indicated by a Roman numeral in parentheses following the name of the metal Fet:iron(II)ion Cu:Copper(1)ion Fe iron(III ion Cu2+:Copper(I)ion -ous the lower An older method: charged Fe2:ferrous ion Cu'cuprous ion -ic:the higher Fe:ferric ion Cu*cupric ion chargedPositive ions--Cations 2. If a metal can form different cations, the positive charge is indicated by a Roman numeral in parentheses following the name of the metal 2 3 : ( ) : ( ) Fe iron II ion Fe iron III ion + 2 : C ( ) : ( ) Cu opper I ion Cu Copper II ion -ous : the lower charged -ic : the higher charged An older method : Rules for Naming Monatomic Ions 2 3 : Fe ferr ion : Fe fer ous r n ic io 2 : : Cu cupr ion Cu cup ous r n ic io