正在加载图片...
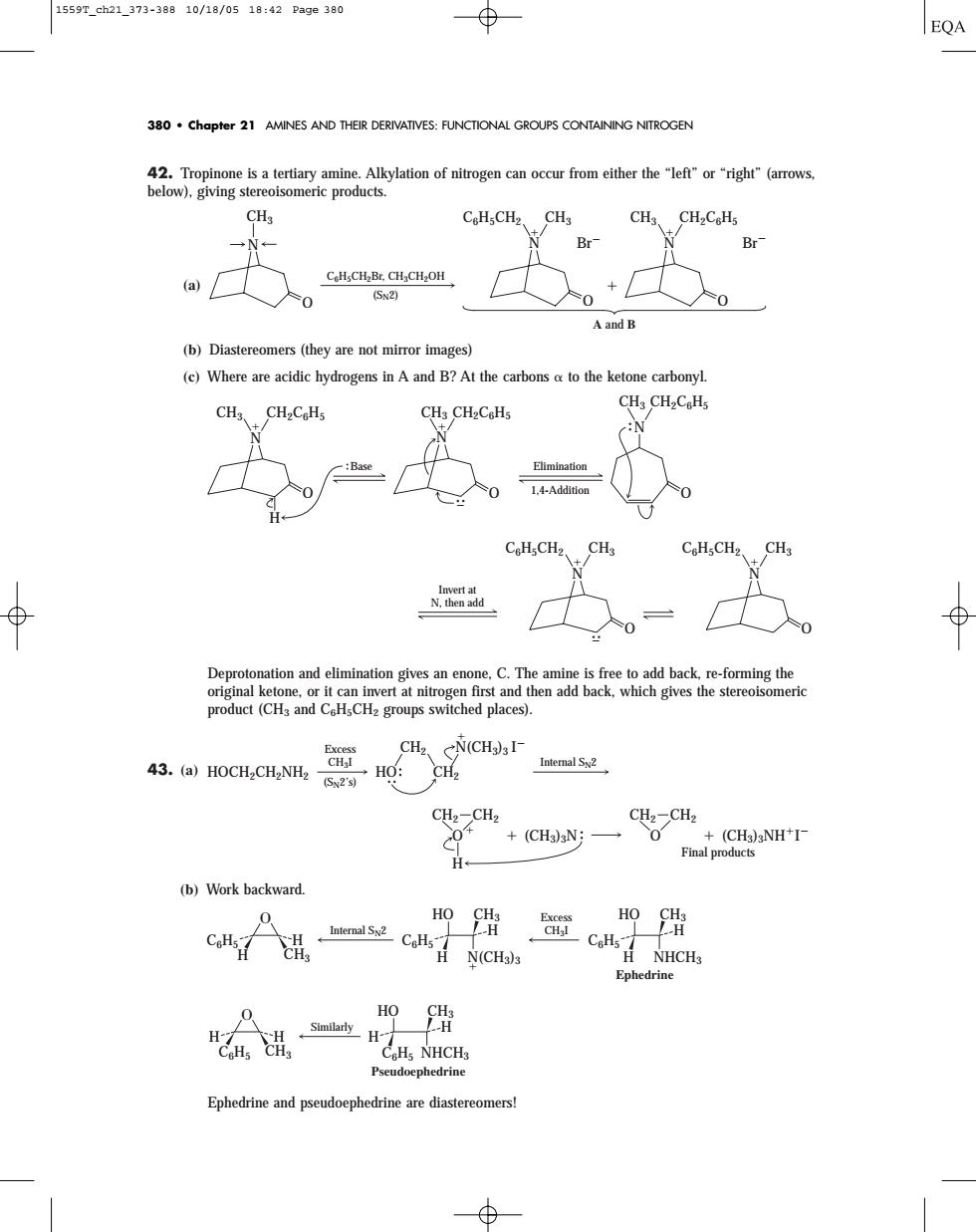
15597.ch21.373-38810/18/0518:42Page380 380.chapter 21 AMINES AND THEIR DERIVATIVES:FUNCTIONAL GROUPS CONTAINING NITROGEN CH CH、CH2CeH + (b)Diastereomers (they are not mirror images) (c)Where are acidic hydrogens in A and B?At the carbons a to the ketone carbonyl. 、CH2C&H. 1.: oH,CH2、CH C.H.CH CH product(CHs and CHsCH2 groups switched places). CH、cN(CHI 43.(a)HOCH2CH2NHz- HO:CH2 Intemal Sx2 (Sy2's) CH2-CH2 CH2-CHz 0 +(CH3N;一 (b)Work backward. HO C 盟兰 CoH,NHCHs42. Tropinone is a tertiary amine. Alkylation of nitrogen can occur from either the “left” or “right” (arrows, below), giving stereoisomeric products. (a) (b) Diastereomers (they are not mirror images) (c) Where are acidic hydrogens in A and B? At the carbons to the ketone carbonyl. Deprotonation and elimination gives an enone, C. The amine is free to add back, re-forming the original ketone, or it can invert at nitrogen first and then add back, which gives the stereoisomeric product (CH3 and C6H5CH2 groups switched places). 43. (a) (b) Work backward. Ephedrine and pseudoephedrine are diastereomers! C6H5 CH3 H H Similarly C6H5 CH3 H H HO NHCH3 Pseudoephedrine C6H5 H CH3 H Excess Internal SN2 CH3I N(CH3)3 C6H5 CH3 H H HO NHCH3 C6H5 CH3 H H HO Ephedrine O CH2 (CH3)3N (CH3)3NHI CH2 O CH2 CH2 H Final products HOCH2CH2NH2 Excess CH3I (SN2’s) Internal SN2 HO CH2 N(CH3)3 I CH2 C6H5CH2 CH3 N O O Invert at N, then add C6H5CH2 CH3 N CH3 CH2C6H5 N H CH3 CH2C6H5 N O CH3 CH2C6H5 N O O Base Elimination 1,4-Addition C6H5CH2Br, CH3CH2OH (SN2) N O CH3 O O N C6H5CH2 CH3 Br N CH3 CH2C6H5 Br A and B 380 • Chapter 21 AMINES AND THEIR DERIVATIVES: FUNCTIONAL GROUPS CONTAINING NITROGEN 1559T_ch21_373-388 10/18/05 18:42 Page 380