正在加载图片...
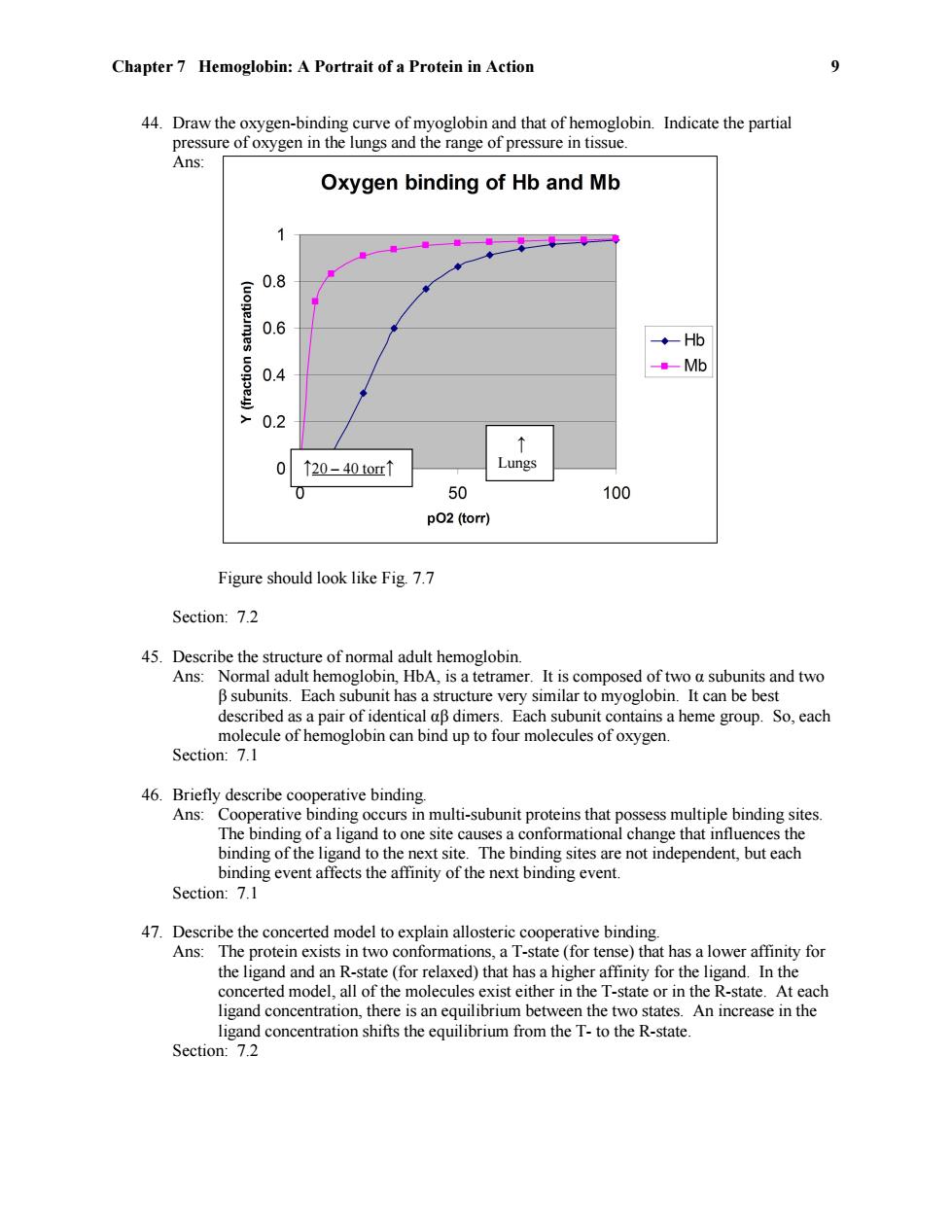
Chapter 7 Hemoglobin:A Portrait of a Protein in Action 9 44.Draw the oxygen-binding curve of myoglobin and that of hemoglobin.Indicate the partial pressure of oxygen in the lungs and the range of pressure in tissue. Ans: Oxygen binding of Hb and Mb 0.8 0.6 ◆Hb 0.4 Mb 0.2 ↑ 0 ↑20-40tor↑ Lungs 0 50 100 p02 (torr) Figure should look like Fig.7.7 Section:7.2 45.Describe the structure of normal adult hemoglobin. Ans:Normal adult hemoglobin,HbA,is a tetramer.It is composed of two a subunits and two B subunits.Each subunit has a structure very similar to myoglobin.It can be best described as a pair of identical aB dimers.Each subunit contains a heme group.So,each molecule of hemoglobin can bind up to four molecules of oxygen. Section:7.1 46.Briefly describe cooperative binding. Ans:Cooperative binding occurs in multi-subunit proteins that possess multiple binding sites. The binding of a ligand to one site causes a conformational change that influences the binding of the ligand to the next site.The binding sites are not independent,but each binding event affects the affinity of the next binding event. Section:7.1 47.Describe the concerted model to explain allosteric cooperative binding. Ans:The protein exists in two conformations,a T-state (for tense)that has a lower affinity for the ligand and an R-state (for relaxed)that has a higher affinity for the ligand.In the concerted model,all of the molecules exist either in the T-state or in the R-state.At each ligand concentration,there is an equilibrium between the two states.An increase in the ligand concentration shifts the equilibrium from the T-to the R-state. Section:7.2Chapter 7 Hemoglobin: A Portrait of a Protein in Action 9 44. Draw the oxygen-binding curve of myoglobin and that of hemoglobin. Indicate the partial pressure of oxygen in the lungs and the range of pressure in tissue. Ans: Figure should look like Fig. 7.7 Section: 7.2 45. Describe the structure of normal adult hemoglobin. Ans: Normal adult hemoglobin, HbA, is a tetramer. It is composed of two α subunits and two β subunits. Each subunit has a structure very similar to myoglobin. It can be best described as a pair of identical αβ dimers. Each subunit contains a heme group. So, each molecule of hemoglobin can bind up to four molecules of oxygen. Section: 7.1 46. Briefly describe cooperative binding. Ans: Cooperative binding occurs in multi-subunit proteins that possess multiple binding sites. The binding of a ligand to one site causes a conformational change that influences the binding of the ligand to the next site. The binding sites are not independent, but each binding event affects the affinity of the next binding event. Section: 7.1 47. Describe the concerted model to explain allosteric cooperative binding. Ans: The protein exists in two conformations, a T-state (for tense) that has a lower affinity for the ligand and an R-state (for relaxed) that has a higher affinity for the ligand. In the concerted model, all of the molecules exist either in the T-state or in the R-state. At each ligand concentration, there is an equilibrium between the two states. An increase in the ligand concentration shifts the equilibrium from the T- to the R-state. Section: 7.2 ↑ Lungs ↑20 – 40 torr↑