
Chapter 7 Hemoglobin:A Portrait of a Protein in Action Matching Questions Use the following to answer questions 1-10: Choose the correct answer from the list below.Not all of the answers will be used a)cooperative b)Bohr effect c)thalassemia d)carbamate e)metmyoglobin f)superoxide g)myoglobin h)bicarbonate ion i)sickle-cell anemia j)protoporphyrin k)fetal 1)carbonic acid This is a reactive oxygen species that is damaging to biological materials. Ans:f Section:7.1 2 This is the organic portion of the heme group in hemoglobin. Ans:j Section:7.1 This is a genetic disease due to the decreased production of one of the subunits of hemoglobin. Ans:c Section:7.4 This is the chemical form in which most of the carbon dioxide is transported in the blood Ans:h Section:7.3 5 This substance is produced when carbon dioxide reacts with water. Ans:I Section:7.3
Chapter 7 Hemoglobin: A Portrait of a Protein in Action Matching Questions Use the following to answer questions 1-10: Choose the correct answer from the list below. Not all of the answers will be used. a) cooperative b) Bohr effect c) thalassemia d) carbamate e) metmyoglobin f) superoxide g) myoglobin h) bicarbonate ion i) sickle-cell anemia j) protoporphyrin k) fetal l) carbonic acid 1 ____________ This is a reactive oxygen species that is damaging to biological materials. Ans: f Section: 7.1 2 ____________ This is the organic portion of the heme group in hemoglobin. Ans: j Section: 7.1 3 ____________ This is a genetic disease due to the decreased production of one of the subunits of hemoglobin. Ans: c Section: 7.4 4 ____________ This is the chemical form in which most of the carbon dioxide is transported in the blood. Ans: h Section: 7.3 5 ____________ This substance is produced when carbon dioxide reacts with water. Ans: l Section: 7.3

Chapter 7 Hemoglobin:A Portrait of a Protein in Action 2 6 This type of hemoglobin is composed of two a chains and two y chains. Ans:k Section:7.2 7 This is the molecule whose function is to store oxygen is muscle cells. Ans:g Section:Introduction This oxidized hemeprotein does not reversibly bind oxygen. Ans:e Section:7.1 9 This type of binding is indicated by a sigmoidal-shaped binding curve. Ans:a Section:7.2 10 This condition is a result of a single point mutation in the B chain of hemoglobin. Ans:i Section:7.4 Fill in the Blank Questions 1 Under normal conditions,the heme iron in myoglobin and hemoglobin is in the oxidation state. Ans:ferrous,or Fe+2 Section:7.1 2 The ability of myoglobin to bind oxygen depends on the presence of a bound prosthetic group called Ans:heme Section:7.1 3 In hemoglobin,the iron of the heme is bonded to the four nitrogens of porphyrin and to the proximal residue of the globin chain. Ans:histidine Section:7.1 4 The binding of 2-3-bisphosphogycerate to hemoglobin (increases,decreases)its affinity of oxygen binding. Ans:decreases Section:7.2 5 The effect of pH on oxygen-binding of hemoglobin is referred to as the Ans:Bohr effect Section:7.3 6 Carbon dioxide reacts with the amino terminal groups of hemoglobin to form carbamate groups, which carry a. charge. Ans:negative Section:7.3
Chapter 7 Hemoglobin: A Portrait of a Protein in Action 2 6 ____________ This type of hemoglobin is composed of two α chains and two γ chains. Ans: k Section: 7.2 7 ____________ This is the molecule whose function is to store oxygen is muscle cells. Ans: g Section: Introduction 8 ____________ This oxidized hemeprotein does not reversibly bind oxygen. Ans: e Section: 7.1 9 ____________ This type of binding is indicated by a sigmoidal-shaped binding curve. Ans: a Section: 7.2 10 ____________ This condition is a result of a single point mutation in the β chain of hemoglobin. Ans: i Section: 7.4 Fill in the Blank Questions 1 Under normal conditions, the heme iron in myoglobin and hemoglobin is in the ____________ oxidation state. Ans: ferrous, or Fe +2 Section: 7.1 2 The ability of myoglobin to bind oxygen depends on the presence of a bound prosthetic group called _____________. Ans: heme Section: 7.1 3 In hemoglobin, the iron of the heme is bonded to the four nitrogens of porphyrin and to the proximal ______________ residue of the globin chain. Ans: histidine Section: 7.1 4 The binding of 2-3-bisphosphogycerate to hemoglobin ____________ (increases, decreases) its affinity of oxygen binding. Ans: decreases Section: 7.2 5 The effect of pH on oxygen-binding of hemoglobin is referred to as the _____________. Ans: Bohr effect Section: 7.3 6 Carbon dioxide reacts with the amino terminal groups of hemoglobin to form carbamate groups, which carry a ______________ charge. Ans: negative Section: 7.3

Chapter 7 Hemoglobin:A Portrait of a Protein in Action 3 7 The T-state of hemoglobin is stabilized by a salt bridge between Bi Asp 94 and the C-terminal of the Bi chain. Ans:histidine Section:7.3 8 In normal adult hemoglobin,HbA,the B6 position is a glutamate residue,whereas in sickle-cell hemoglobin,HbS,it is a residue. Ans:valine Section:7.4 9 As the pressure of carbon increases,the affinity of oxygen binding to hemoglobin Ans:decreases Section:7.3 10 2,3-Bisphosphoglycerate binds only to the form of hemoglobin. Ans:T-,or deoxy Section:7.2 Multiple Choice Questions 1 What factor(s)influence(s)the binding of oxygen to myoglobin? A)The concentration of bicarbonate ion,HCO3 B) The partial pressure of oxygen,pO2 C) The concentration of hemoglobin present D The concentration of 2,3-BPG E) Responses b and d Ans:B Section:7.1 2 Which of the following is correct concerning the differences between hemoglobin and myoglobin? A)Both hemoglobin and myoglobin are tetrameric proteins. B) Hemoglobin exhibits a hyperbolic O2 saturation curve while myoglobin exhibits a sigmoid shaped curve. C) Hemoglobin exhibits cooperative binding of O2 while myoglobin does not. D) Hemoglobin exhibits a higher degree of O2 saturation at all physiologically relevant partial pressures of O2 than does myoglobin. E) All of the above. Ans:C Section:7.1 3 Which of the following is not correct concerning myoglobin? A)The globin chain contains an extensive a-helix structure. B) The heme group is bound to the globin chain by two disulfide bonds to cysteine residues C)The iron of the heme group is in the Fe+2 oxidation state. D)The diameter of the iron ion decreases upon binding to oxygen. E)The function of myoglobin is oxygen storage in muscle. Ans:B Section:7.1
Chapter 7 Hemoglobin: A Portrait of a Protein in Action 3 7 The T-state of hemoglobin is stabilized by a salt bridge between β1 Asp 94 and the C-terminal ___________________ of the β1 chain. Ans: histidine Section: 7.3 8 In normal adult hemoglobin, HbA, the β6 position is a glutamate residue, whereas in sickle-cell hemoglobin, HbS, it is a ____________ residue. Ans: valine Section: 7.4 9 As the pressure of carbon increases, the affinity of oxygen binding to hemoglobin ______________. Ans: decreases Section: 7.3 10 2,3-Bisphosphoglycerate binds only to the __________________ form of hemoglobin. Ans: T-, or deoxy Section: 7.2 Multiple Choice Questions 1 What factor(s) influence(s) the binding of oxygen to myoglobin? A) The concentration of bicarbonate ion, HCO3 - B) The partial pressure of oxygen, pO2 C) The concentration of hemoglobin present D) The concentration of 2,3-BPG E) Responses b and d Ans: B Section: 7.1 2 Which of the following is correct concerning the differences between hemoglobin and myoglobin? A) Both hemoglobin and myoglobin are tetrameric proteins. B) Hemoglobin exhibits a hyperbolic O2 saturation curve while myoglobin exhibits a sigmoid shaped curve. C) Hemoglobin exhibits cooperative binding of O2 while myoglobin does not. D) Hemoglobin exhibits a higher degree of O2 saturation at all physiologically relevant partial pressures of O2 than does myoglobin. E) All of the above. Ans: C Section: 7.1 3 Which of the following is not correct concerning myoglobin? A) The globin chain contains an extensive α-helix structure. B) The heme group is bound to the globin chain by two disulfide bonds to cysteine residues C) The iron of the heme group is in the Fe +2 oxidation state. D) The diameter of the iron ion decreases upon binding to oxygen. E) The function of myoglobin is oxygen storage in muscle. Ans: B Section: 7.1

Chapter 7 Hemoglobin:A Portrait of a Protein in Action 4 4 The structure of normal adult hemoglobin can be described as A)a tetramer composed of four myoglobin molecules. B) a tetramer composed of two aB dimmers. c) a tetramer composed of two a2 and two B2 dimers. D) a tetramer composed of two a2 and two y2 dimers. E)None of these accurately describe hemoglobin. Ans:B Section:7.1 5 Which of the following is correct concerning fetal hemoglobin? A)Fetal hemoglobin is composed of two a and two y subunits B)Fetal hemoglobin binds 2,3-BPG more tightly than normal adult hemoglobin. C) Fetal hemoglobin binds oxygen less than HbA at all pO2. D) Fetal hemoglobin does not exist in the T-form. E)None of the above. Ans:A Section:7.2 26.Hemoglobin-binding of oxygen is best described as a A) concerted model. B) Michaelis-Menten model. C) sequential model. D) combination of sequential and concerted models E) None of the above. Ans:D Section:7.2 27.2-3 Bisphosphoglycerate A) binds in the central cavity in the T-form of hemoglobin. B) preferentially binds to deoxyhemoglobin and stabilizes it. C) is present in the red blood cells. D) All of the above. E)None of the above. Ans:D Section:7.2 28.What is the Bohr effect? A)the ability of hemoglobin to retain oxygen when in competition with myoglobin B)the regulation of hemoglobin-binding by hydrogen ions and carbon dioxide C) the alteration of hemoglobin conformation during low oxygen stress D) All of the above. E)None of the above. Ans:B Section:7.3 29.Which of the following statements is correct for hemoglobin and oxygen transport? A)The oxygen binds to the proximal histidine residue of the globin chain B) Bonding of carbon dioxide to hemoglobin molecules increases the binding of oxygen. C) Hemoglobin binds more oxygen as the pH is lowered. D) Hemoglobin binds more oxygen at higher [BPG]concentrations. E)The binding of each O2 molecule to hemoglobin increases its affinity for the next O2 Ans:E Section:7.1
Chapter 7 Hemoglobin: A Portrait of a Protein in Action 4 4 The structure of normal adult hemoglobin can be described as A) a tetramer composed of four myoglobin molecules. B) a tetramer composed of two αβ dimmers. C) a tetramer composed of two α2 and two β2 dimers. D) a tetramer composed of two α2 and two γ2 dimers. E) None of these accurately describe hemoglobin. Ans: B Section: 7.1 5 Which of the following is correct concerning fetal hemoglobin? A) Fetal hemoglobin is composed of two α and two γ subunits. B) Fetal hemoglobin binds 2,3-BPG more tightly than normal adult hemoglobin. C) Fetal hemoglobin binds oxygen less than HbA at all pO2. D) Fetal hemoglobin does not exist in the T-form. E) None of the above. Ans: A Section: 7.2 26. Hemoglobin-binding of oxygen is best described as a A) concerted model. B) Michaelis-Menten model. C) sequential model. D) combination of sequential and concerted models. E) None of the above. Ans: D Section: 7.2 27. 2-3 Bisphosphoglycerate A) binds in the central cavity in the T-form of hemoglobin. B) preferentially binds to deoxyhemoglobin and stabilizes it. C) is present in the red blood cells. D) All of the above. E) None of the above. Ans: D Section: 7.2 28. What is the Bohr effect? A) the ability of hemoglobin to retain oxygen when in competition with myoglobin B) the regulation of hemoglobin-binding by hydrogen ions and carbon dioxide C) the alteration of hemoglobin conformation during low oxygen stress D) All of the above. E) None of the above. Ans: B Section: 7.3 29. Which of the following statements is correct for hemoglobin and oxygen transport? A) The oxygen binds to the proximal histidine residue of the globin chain. B) Bonding of carbon dioxide to hemoglobin molecules increases the binding of oxygen. C) Hemoglobin binds more oxygen as the pH is lowered. D) Hemoglobin binds more oxygen at higher [BPG] concentrations. E) The binding of each O2 molecule to hemoglobin increases its affinity for the next O2 . Ans: E Section: 7.1

Chapter 7 Hemoglobin:A Portrait of a Protein in Action 30.Which of the following describes the Bohr Effect? A) Lowering the pH results in the release of O2 from oxyhemoglobin. B) Increasing the pressure of CO2 results in the release of O2 from oxyhemoglobin. c) Increasing the pH increases the T-form of hemoglobin. D) All of the above. E)a and b Ans:E Section:7.3 31.Which of the following is correct concerning the following equilibria? C02+H20=H2CO3 A)An increase in the pressure of CO2 will result in a decrease of pH. B This reaction is catalyzed by carbonic anhydrase. C) The H2CO3 dissociates to H'and bicarbonate ion,HCO3. D) The majority of CO2 is transported to the lungs in the form of HCO3. E) All of the above. Ans:E Section:7.3 32.Carbon dioxide forms carbamate groups in proteins by reaction with A)aspartate residues. B) cysteine residues. C) N-terminal amino groups. D) tyrosine residues. E) heme groups. Ans:C Section:7.3 33.Sickle-cell anemia is caused by A) a decreased production of a chains of hemoglobin. B) a substitution of a Glu residue for a Phe residue at the B6 position. C)the loss of the heme group because the proximal His is oxidized. D) a substitution of a Val residue for a Glu residue at the B6 position. E)a substitution of Glu residue for His at the C-terminal of the a chain. Ans:D Section:7.4
Chapter 7 Hemoglobin: A Portrait of a Protein in Action 5 30. Which of the following describes the Bohr Effect? A) Lowering the pH results in the release of O2 from oxyhemoglobin. B) Increasing the pressure of CO2 results in the release of O2 from oxyhemoglobin. C) Increasing the pH increases the T-form of hemoglobin. D) All of the above. E) a and b Ans: E Section: 7.3 31. Which of the following is correct concerning the following equilibria? CO2 + H2O H2CO3 A) An increase in the pressure of CO2 will result in a decrease of pH. B) This reaction is catalyzed by carbonic anhydrase. C) The H2CO3 dissociates to H+ and bicarbonate ion, HCO3 -. D) The majority of CO2 is transported to the lungs in the form of HCO3 -. E) All of the above. Ans: E Section: 7.3 32. Carbon dioxide forms carbamate groups in proteins by reaction with A) aspartate residues. B) cysteine residues. C) N-terminal amino groups. D) tyrosine residues. E) heme groups. Ans: C Section: 7.3 33. Sickle-cell anemia is caused by A) a decreased production of α chains of hemoglobin. B) a substitution of a Glu residue for a Phe residue at the β6 position. C) the loss of the heme group because the proximal His is oxidized. D) a substitution of a Val residue for a Glu residue at the β6 position. E) a substitution of Glu residue for His at the C-terminal of the α chain. Ans: D Section: 7.4
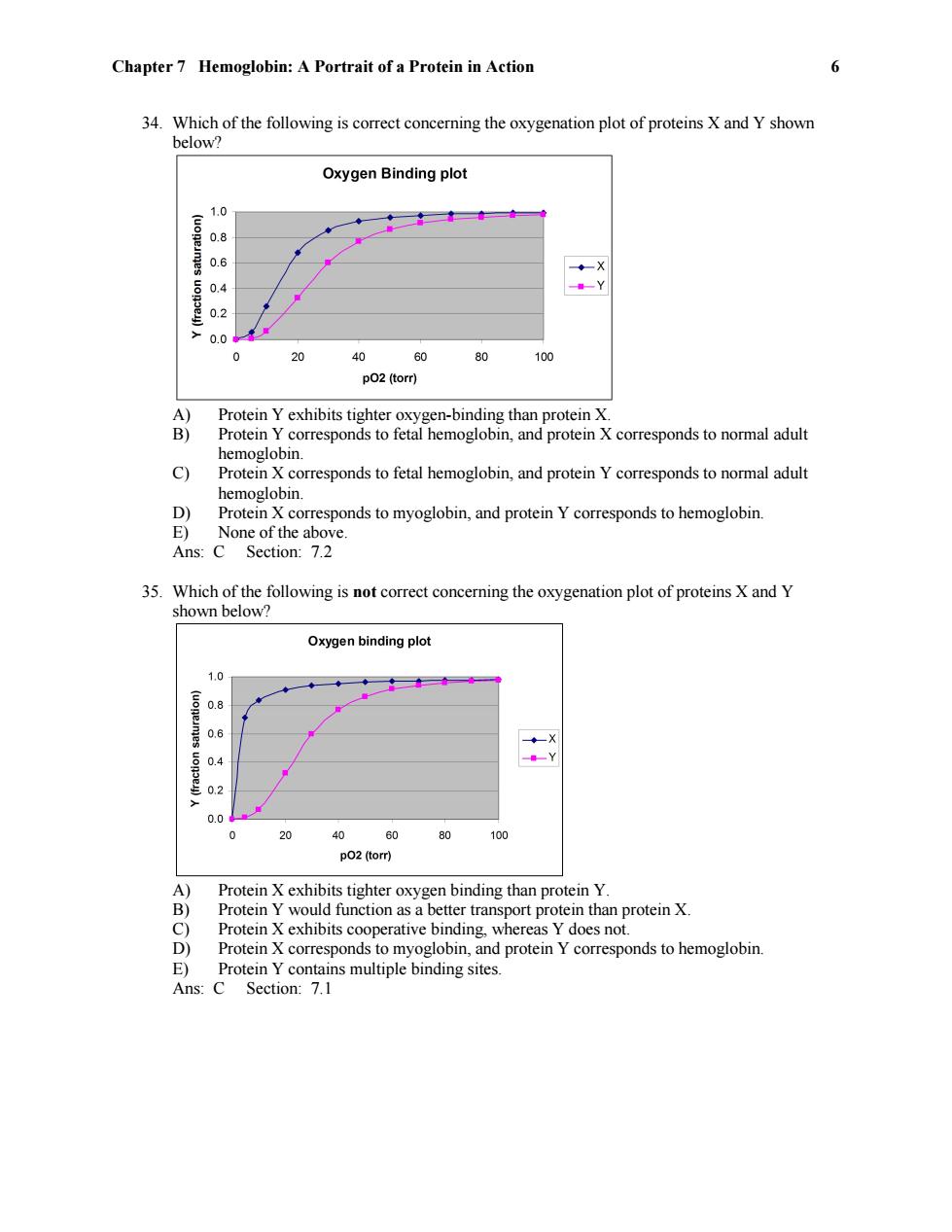
Chapter 7 Hemoglobin:A Portrait of a Protein in Action 6 34.Which of the following is correct concerning the oxygenation plot of proteins X and Y shown below? Oxygen Binding plot 10 (uoneimes uonoey) 08 -Y 0.2 0.0 0 20 40 60 80 100 p02(torr) A) Protein Y exhibits tighter oxygen-binding than protein X. B) Protein Y corresponds to fetal hemoglobin,and protein X corresponds to normal adult hemoglobin. c) Protein X corresponds to fetal hemoglobin,and protein Y corresponds to normal adult hemoglobin. D) Protein X corresponds to myoglobin,and protein Y corresponds to hemoglobin. E) None of the above. Ans:C Section:7.2 35. Which of the following is not correct concerning the oxygenation plot of proteins X and Y shown below? Oxygen binding plot 1.0 0.6 ◆一X 02 0.0 0 20 40 60 80 100 p02(torr) A) Protein X exhibits tighter oxygen binding than protein Y. B) Protein Y would function as a better transport protein than protein X. C) Protein X exhibits cooperative binding,whereas Y does not D) Protein X corresponds to myoglobin,and protein Y corresponds to hemoglobin. E) Protein Y contains multiple binding sites. Ans:C Section:7.1
Chapter 7 Hemoglobin: A Portrait of a Protein in Action 6 34. Which of the following is correct concerning the oxygenation plot of proteins X and Y shown below? A) Protein Y exhibits tighter oxygen-binding than protein X. B) Protein Y corresponds to fetal hemoglobin, and protein X corresponds to normal adult hemoglobin. C) Protein X corresponds to fetal hemoglobin, and protein Y corresponds to normal adult hemoglobin. D) Protein X corresponds to myoglobin, and protein Y corresponds to hemoglobin. E) None of the above. Ans: C Section: 7.2 35. Which of the following is not correct concerning the oxygenation plot of proteins X and Y shown below? A) Protein X exhibits tighter oxygen binding than protein Y. B) Protein Y would function as a better transport protein than protein X. C) Protein X exhibits cooperative binding, whereas Y does not. D) Protein X corresponds to myoglobin, and protein Y corresponds to hemoglobin. E) Protein Y contains multiple binding sites. Ans: C Section: 7.1
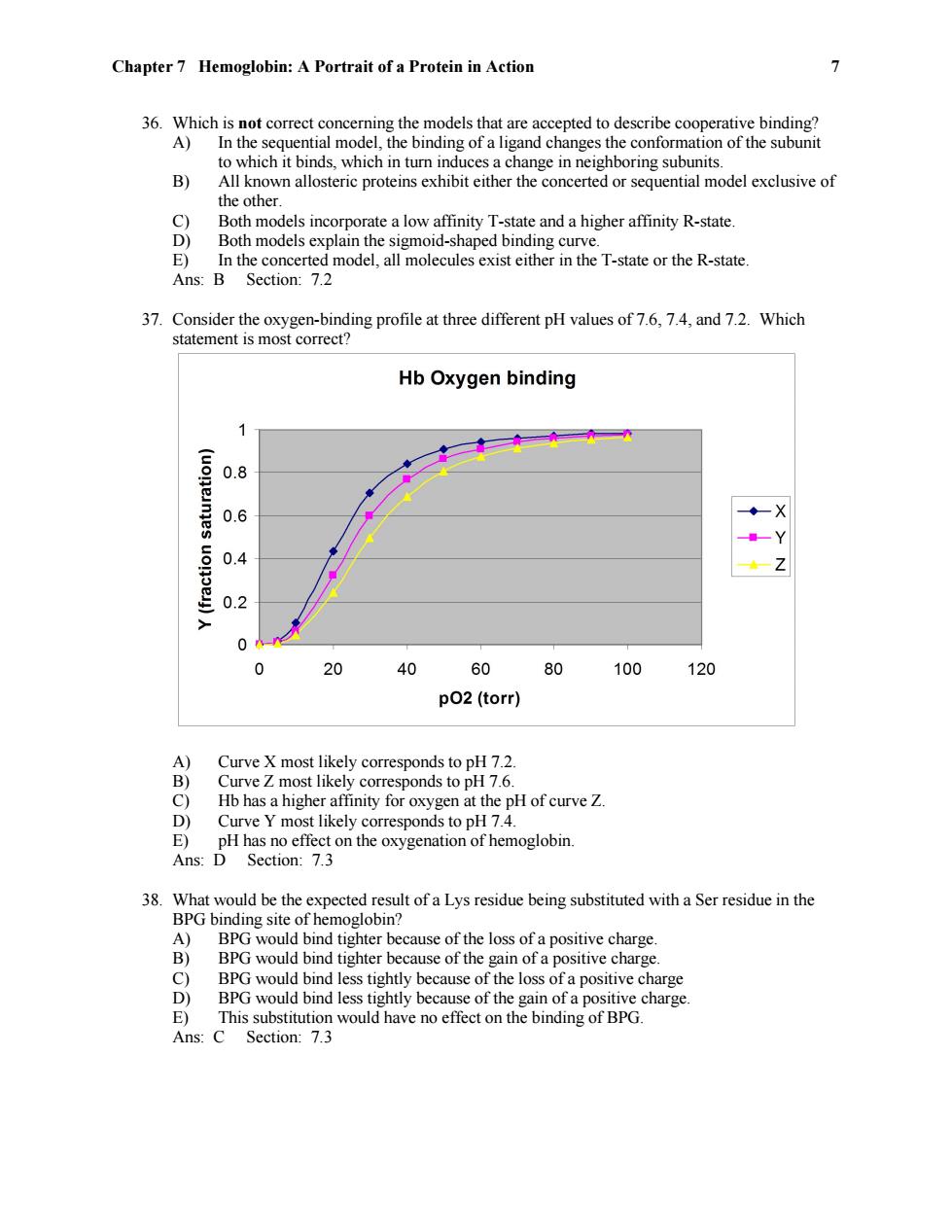
Chapter 7 Hemoglobin:A Portrait of a Protein in Action 7 36.Which is not correct concerning the models that are accepted to describe cooperative binding? A)In the sequential model,the binding of a ligand changes the conformation of the subunit to which it binds,which in turn induces a change in neighboring subunits. B) All known allosteric proteins exhibit either the concerted or sequential model exclusive of the other. C) Both models incorporate a low affinity T-state and a higher affinity R-state D) Both models explain the sigmoid-shaped binding curve. E)In the concerted model,all molecules exist either in the T-state or the R-state Ans:B Section:7.2 37.Consider the oxygen-binding profile at three different pH values of 7.6,7.4,and 7.2.Which statement is most correct? Hb Oxygen binding 0.8 0.6 0.4 Z 0.2 0 20 40 60 80 100 120 p02(torr) A) Curve X most likely corresponds to pH 7.2 B) Curve Z most likely corresponds to pH 7.6. C) Hb has a higher affinity for oxygen at the pH of curve Z. D) Curve Y most likely corresponds to pH 7.4. E)pH has no effect on the oxygenation of hemoglobin. Ans:D Section:7.3 38.What would be the expected result of a Lys residue being substituted with a Ser residue in the BPG binding site of hemoglobin? A)BPG would bind tighter because of the loss of a positive charge B) BPG would bind tighter because of the gain of a positive charge. C) BPG would bind less tightly because of the loss of a positive charge D) BPG would bind less tightly because of the gain of a positive charge. E)This substitution would have no effect on the binding of BPG Ans:C Section:7.3
Chapter 7 Hemoglobin: A Portrait of a Protein in Action 7 36. Which is not correct concerning the models that are accepted to describe cooperative binding? A) In the sequential model, the binding of a ligand changes the conformation of the subunit to which it binds, which in turn induces a change in neighboring subunits. B) All known allosteric proteins exhibit either the concerted or sequential model exclusive of the other. C) Both models incorporate a low affinity T-state and a higher affinity R-state. D) Both models explain the sigmoid-shaped binding curve. E) In the concerted model, all molecules exist either in the T-state or the R-state. Ans: B Section: 7.2 37. Consider the oxygen-binding profile at three different pH values of 7.6, 7.4, and 7.2. Which statement is most correct? A) Curve X most likely corresponds to pH 7.2. B) Curve Z most likely corresponds to pH 7.6. C) Hb has a higher affinity for oxygen at the pH of curve Z. D) Curve Y most likely corresponds to pH 7.4. E) pH has no effect on the oxygenation of hemoglobin. Ans: D Section: 7.3 38. What would be the expected result of a Lys residue being substituted with a Ser residue in the BPG binding site of hemoglobin? A) BPG would bind tighter because of the loss of a positive charge. B) BPG would bind tighter because of the gain of a positive charge. C) BPG would bind less tightly because of the loss of a positive charge D) BPG would bind less tightly because of the gain of a positive charge. E) This substitution would have no effect on the binding of BPG. Ans: C Section: 7.3

Chapter 7 Hemoglobin:A Portrait of a Protein in Action 8 Short-Answer Questions 39.Why is it advantageous for hemoglobin to have allosteric properties? Ans:Hemoglobin binds oxygen in a positive cooperative manner.This allows it to become saturated in the lungs,where oxygen pressure is high.When the hemoglobin moves to tissues,the lower oxygen pressure induces it to release oxygen and thus deliver oxygen where it is needed. Section:7.1 40.What is fetal hemoglobin?How does it differ from adult hemoglobin? Ans:Fetal hemoglobin contains two a and two y chains,in contrast to adult hemoglobin with two a and two B chains.The fetal hemoglobiny chain is probably a result of gene duplication and divergence.The difference in the chains results in a lower binding affinity of 2-3 BPG to fetal hemoglobin.Thus,the fetal hemoglobin has a higher affinity for oxygen,and the oxygen is effectively transferred from the mother's hemoglobin to fetal hemoglobin. Section:7.2 41.What is metmyoglobin? Ans:Metmyoglobin is formed when the heme iron ion,which is normally in the +2 oxidation state,is oxidized to the +3 oxidation state.This oxidized form of myoglobin does not bind dioxygen and is not functional. Section:7.1 42.Describe the octahedral coordination sphere of the iron ion in hemoglobin and myoglobin. Ans:The Fe+2 ion is coordinated to the four nitrogens in the center of the protoporphyrin of the heme.The fifth coordination site is occupied by the "proximal histidine"of the globin chain.The oxygen is bound to the sixth coordination site of the iron. Section:7.1 43.What functional role does the"distal histidine"play in the function of myoglobin and hemoglobin? Ans:The bonding between the iron and oxygen can be described as a combination of resonance structures,one with Fe2+and dioxygen and another with Fe and superoxide. The"distal histidine"donates a hydrogen bond to this complex stabilizing the complex and inhibits the oxidation of the iron to the ferric state. Section:7.1
Chapter 7 Hemoglobin: A Portrait of a Protein in Action 8 Short-Answer Questions 39. Why is it advantageous for hemoglobin to have allosteric properties? Ans: Hemoglobin binds oxygen in a positive cooperative manner. This allows it to become saturated in the lungs, where oxygen pressure is high. When the hemoglobin moves to tissues, the lower oxygen pressure induces it to release oxygen and thus deliver oxygen where it is needed. Section: 7.1 40. What is fetal hemoglobin? How does it differ from adult hemoglobin? Ans: Fetal hemoglobin contains two and two chains, in contrast to adult hemoglobin with two and two chains. The fetal hemoglobin chain is probably a result of gene duplication and divergence. The difference in the chains results in a lower binding affinity of 2-3 BPG to fetal hemoglobin. Thus, the fetal hemoglobin has a higher affinity for oxygen, and the oxygen is effectively transferred from the mother’s hemoglobin to fetal hemoglobin. Section: 7.2 41. What is metmyoglobin? Ans: Metmyoglobin is formed when the heme iron ion, which is normally in the +2 oxidation state, is oxidized to the +3 oxidation state. This oxidized form of myoglobin does not bind dioxygen and is not functional. Section: 7.1 42. Describe the octahedral coordination sphere of the iron ion in hemoglobin and myoglobin. Ans: The Fe +2 ion is coordinated to the four nitrogens in the center of the protoporphyrin of the heme. The fifth coordination site is occupied by the “proximal histidine” of the globin chain. The oxygen is bound to the sixth coordination site of the iron. Section: 7.1 43. What functional role does the “distal histidine” play in the function of myoglobin and hemoglobin? Ans: The bonding between the iron and oxygen can be described as a combination of resonance structures, one with Fe 2+ and dioxygen and another with Fe 3+ and superoxide. The “distal histidine” donates a hydrogen bond to this complex stabilizing the complex and inhibits the oxidation of the iron to the ferric state. Section: 7.1
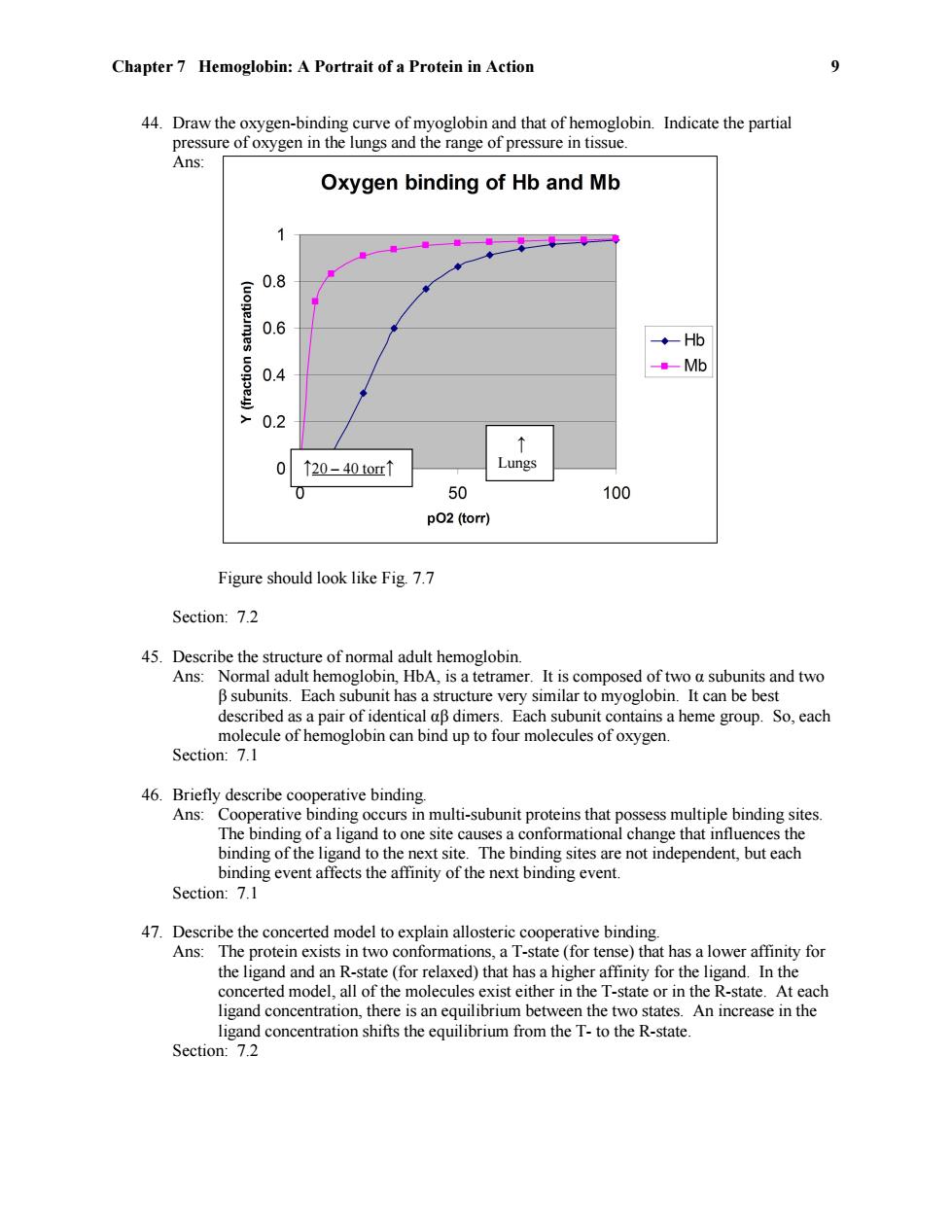
Chapter 7 Hemoglobin:A Portrait of a Protein in Action 9 44.Draw the oxygen-binding curve of myoglobin and that of hemoglobin.Indicate the partial pressure of oxygen in the lungs and the range of pressure in tissue. Ans: Oxygen binding of Hb and Mb 0.8 0.6 ◆Hb 0.4 Mb 0.2 ↑ 0 ↑20-40tor↑ Lungs 0 50 100 p02 (torr) Figure should look like Fig.7.7 Section:7.2 45.Describe the structure of normal adult hemoglobin. Ans:Normal adult hemoglobin,HbA,is a tetramer.It is composed of two a subunits and two B subunits.Each subunit has a structure very similar to myoglobin.It can be best described as a pair of identical aB dimers.Each subunit contains a heme group.So,each molecule of hemoglobin can bind up to four molecules of oxygen. Section:7.1 46.Briefly describe cooperative binding. Ans:Cooperative binding occurs in multi-subunit proteins that possess multiple binding sites. The binding of a ligand to one site causes a conformational change that influences the binding of the ligand to the next site.The binding sites are not independent,but each binding event affects the affinity of the next binding event. Section:7.1 47.Describe the concerted model to explain allosteric cooperative binding. Ans:The protein exists in two conformations,a T-state (for tense)that has a lower affinity for the ligand and an R-state (for relaxed)that has a higher affinity for the ligand.In the concerted model,all of the molecules exist either in the T-state or in the R-state.At each ligand concentration,there is an equilibrium between the two states.An increase in the ligand concentration shifts the equilibrium from the T-to the R-state. Section:7.2
Chapter 7 Hemoglobin: A Portrait of a Protein in Action 9 44. Draw the oxygen-binding curve of myoglobin and that of hemoglobin. Indicate the partial pressure of oxygen in the lungs and the range of pressure in tissue. Ans: Figure should look like Fig. 7.7 Section: 7.2 45. Describe the structure of normal adult hemoglobin. Ans: Normal adult hemoglobin, HbA, is a tetramer. It is composed of two α subunits and two β subunits. Each subunit has a structure very similar to myoglobin. It can be best described as a pair of identical αβ dimers. Each subunit contains a heme group. So, each molecule of hemoglobin can bind up to four molecules of oxygen. Section: 7.1 46. Briefly describe cooperative binding. Ans: Cooperative binding occurs in multi-subunit proteins that possess multiple binding sites. The binding of a ligand to one site causes a conformational change that influences the binding of the ligand to the next site. The binding sites are not independent, but each binding event affects the affinity of the next binding event. Section: 7.1 47. Describe the concerted model to explain allosteric cooperative binding. Ans: The protein exists in two conformations, a T-state (for tense) that has a lower affinity for the ligand and an R-state (for relaxed) that has a higher affinity for the ligand. In the concerted model, all of the molecules exist either in the T-state or in the R-state. At each ligand concentration, there is an equilibrium between the two states. An increase in the ligand concentration shifts the equilibrium from the T- to the R-state. Section: 7.2 ↑ Lungs ↑20 – 40 torr↑

Chapter 7 Hemoglobin:A Portrait of a Protein in Action 10 48.Describe the role of 2,3-bisphosphoglycerate in the function of hemoglobin. Ans:2.3-bisphosphoglycerate,2,3-BPG,is a relatively small,highly anionic molecule found in the RBC.2,3-BPG only binds to the center cavity of deoxyhemoglobin(T-state).The size of the center cavity decreases upon the change to the R-form so that it cannot bind to the R-state.Thus,the presence of 2,3-BPG shifts the equilibrium toward the T-state.T- state is unstable.and without BPG.the equilibrium shifts so far toward the R-state that little oxygen would be released under physiological conditions. Section:7.2 49.Describe the chemical basis of the Bohr effect. Ans:The effect observed by Christian Bohr is that hemoglobin becomes deoxygenated as the pH decreases.In deoxyhemoglobin,three amino acid residues form two salt bridges that stabilize the T-state.One of these is formed between the C-terminal His B146 and an Asp residue(B94).As the pH increases,this stabilizing salt bridge is broken because His becomes deprotonated and loses its positive charge.At lower pH values,this His is positively charged.The formation of the salt bridge shifts the equilibrium from the R- state to the T-state,thus releasing oxygen. Section:7.3 50.Describe how carbon dioxide affects the oxygenation of hemoglobin. Ans:Increased levels of carbon dioxide cause hemoglobin to release oxygen.The more active the tissue,the more fuel is burned and the more CO2 is produced.These active tissue cells have the greatest need for oxygen to produce more energy.The CO2 combines with the N-terminal amino groups to form negatively charge carbamate groups.The negatively charge carbamate groups form salt bridges that stabilize the T-state.Thus,the increase of carbon dioxide causes the conversion of the R-state to the T-state,releasing the bound oxygen to the tissues producing the most CO2. Section:7.3 51.Briefly describe the cause of sickle-cell anemia. Ans:Sickle-cell anemia is a genetic disorder that is the result of a single substitution of B6 Glu with a Val.This changes a negatively charged side chain to a nonpolar,hydrophobic side chain.This Val binds into a hydrophobic pocket on the B chain of an adjacent molecule whose B6 Val binds to another molecule,thus hemoglobin aggregates.These aggregates form long fibers that strain the RBC and force into a sickled shape.The distorted red blood cells clog capillaries and impair blood flow,resulting in the sickle-cell crisis.The sickled cells are then destroyed,resulting in the anemia. Section:7.4 52.What is thalassemia? Ans:Thalassemia is caused by the substantial decreased production of one of the subunits of hemoglobin.In a-thalassemia,the decreased production of the a chain results in the formation of tetramers of only the B chain.This B4 binds oxygen more tightly than HbA and does not exhibit cooperative binding.In B-thalassemia,the a chains form insoluble aggregates in the immature red blood cells. Section:7.4
Chapter 7 Hemoglobin: A Portrait of a Protein in Action 10 48. Describe the role of 2,3-bisphosphoglycerate in the function of hemoglobin. Ans: 2,3-bisphosphoglycerate, 2,3-BPG, is a relatively small, highly anionic molecule found in the RBC. 2,3-BPG only binds to the center cavity of deoxyhemoglobin (T-state). The size of the center cavity decreases upon the change to the R-form so that it cannot bind to the R-state. Thus, the presence of 2,3-BPG shifts the equilibrium toward the T-state. T- state is unstable, and without BPG, the equilibrium shifts so far toward the R-state that little oxygen would be released under physiological conditions. Section: 7.2 49. Describe the chemical basis of the Bohr effect. Ans: The effect observed by Christian Bohr is that hemoglobin becomes deoxygenated as the pH decreases. In deoxyhemoglobin, three amino acid residues form two salt bridges that stabilize the T-state. One of these is formed between the C-terminal His β146 and an Asp residue (β94). As the pH increases, this stabilizing salt bridge is broken because His becomes deprotonated and loses its positive charge. At lower pH values, this His is positively charged. The formation of the salt bridge shifts the equilibrium from the R- state to the T-state, thus releasing oxygen. Section: 7.3 50. Describe how carbon dioxide affects the oxygenation of hemoglobin. Ans: Increased levels of carbon dioxide cause hemoglobin to release oxygen. The more active the tissue, the more fuel is burned and the more CO2 is produced. These active tissue cells have the greatest need for oxygen to produce more energy. The CO2 combines with the N-terminal amino groups to form negatively charge carbamate groups. The negatively charge carbamate groups form salt bridges that stabilize the T-state. Thus, the increase of carbon dioxide causes the conversion of the R-state to the T-state, releasing the bound oxygen to the tissues producing the most CO2. Section: 7.3 51. Briefly describe the cause of sickle-cell anemia. Ans: Sickle-cell anemia is a genetic disorder that is the result of a single substitution of β6 Glu with a Val. This changes a negatively charged side chain to a nonpolar, hydrophobic side chain. This Val binds into a hydrophobic pocket on the β chain of an adjacent molecule whose β6 Val binds to another molecule, thus hemoglobin aggregates. These aggregates form long fibers that strain the RBC and force into a sickled shape. The distorted red blood cells clog capillaries and impair blood flow, resulting in the sickle-cell crisis. The sickled cells are then destroyed, resulting in the anemia. Section: 7.4 52. What is thalassemia? Ans: Thalassemia is caused by the substantial decreased production of one of the subunits of hemoglobin. In α-thalassemia, the decreased production of the α chain results in the formation of tetramers of only the β chain. This β4 binds oxygen more tightly than HbA and does not exhibit cooperative binding. In β-thalassemia, the α chains form insoluble aggregates in the immature red blood cells. Section: 7.4