
Chapter 11 Carbohydrates Matching Questions Use the following to answer questions 1-10: Choose the correct answer from the list below.Not all of the answers will be used. a)Fehling's b)enantiomers e)UDP d)glycogen e)monosaccharides f)cellulose g)lectins h)Heparin i)axial j)glycoproteins k)epimers 1)glycosyltransferases This class of compounds has the molecular formula of(CH2O)n. Ans:e Section:11.1 2 These are stereoisomers that are mirror images of each other. Ans:b Section:11.1 These monosaccharides differ at a single asymmetric carbon. Ans:k Section:11.1 4 This is the most abundant organic molecule in the biosphere. Ans:f Section:11.2 5 This is a test solution used to identify reducing and nonreducing sugars. Ans:a Section:11.1 6 The storage form of glucose in animals. Ans:d Section:11.2
Chapter 11 Carbohydrates Matching Questions Use the following to answer questions 1-10: Choose the correct answer from the list below. Not all of the answers will be used. a) Fehling’s b) enantiomers c) UDP d) glycogen e) monosaccharides f) cellulose g) lectins h) Heparin i) axial j) glycoproteins k) epimers l) glycosyltransferases 1 ____________ This class of compounds has the molecular formula of (CH2O)n. Ans: e Section: 11.1 2 ____________ These are stereoisomers that are mirror images of each other. Ans: b Section: 11.1 3 ____________ These monosaccharides differ at a single asymmetric carbon. Ans: k Section: 11.1 4 ____________ This is the most abundant organic molecule in the biosphere. Ans: f Section: 11.2 5 ____________ This is a test solution used to identify reducing and nonreducing sugars. Ans: a Section: 11.1 6 ____________ The storage form of glucose in animals. Ans: d Section: 11.2

Chapter 11 Carbohydrates 2 7 This is an example of a glycosaminoglycan. Ans:h Section:11.2 8 These are the enzymes that synthesize oligosaccharides. Ans:I Section:11.2 9 Molecule to which most sugars are attached prior to transfer. Ans:c Section:11.2 10 These proteins bind to specific carbohydrate structures. Ans:g Section:11.3 Fill in the Blank Questions 1A is a stereoisomer that is not a mirror image. Ans:diastereoisomer Section:11.1 2A is a five-membered ring formed from a monosaccharide. Ans:furanose Section:11.1 3A is formed when two monosaccharides are linked together via a glycosidic bond. Ans:disaccharide Section:11.1 4 Plant starch is composed of amylose,a linear polymer of glucose,and a branched polymer of glucose referred to as Ans:amylopectin Section:11.2 5 Maltose is composed of two molecules of glucose linked together by glycosidic bond. Ans:a-1,4 Section:11.2 6 is a galactose joined to a glucose by a B-1,4 glycosidic bond. Ans:Lactose Section:11.2 7 In N-linked glycoproteins,the carbohydrate portion is attached to a(n). residue in the protein. Ans:asparagine Section:11.3
Chapter 11 Carbohydrates 2 7 ____________ This is an example of a glycosaminoglycan. Ans: h Section: 11.2 8 ____________ These are the enzymes that synthesize oligosaccharides. Ans: l Section: 11.2 9 ____________ Molecule to which most sugars are attached prior to transfer. Ans: c Section: 11.2 10 ____________ These proteins bind to specific carbohydrate structures. Ans: g Section: 11.3 Fill in the Blank Questions 1 A _______________ is a stereoisomer that is not a mirror image. Ans: diastereoisomer Section: 11.1 2 A _______________ is a five-membered ring formed from a monosaccharide. Ans: furanose Section: 11.1 3 A _______________ is formed when two monosaccharides are linked together via a glycosidic bond. Ans: disaccharide Section: 11.1 4 Plant starch is composed of amylose, a linear polymer of glucose, and a branched polymer of glucose referred to as _______________. Ans: amylopectin Section: 11.2 5 Maltose is composed of two molecules of glucose linked together by ____________ glycosidic bond. Ans: α-1,4 Section: 11.2 6 _______________ is a galactose joined to a glucose by a β-1,4 glycosidic bond. Ans: Lactose Section: 11.2 7 In N-linked glycoproteins, the carbohydrate portion is attached to a(n)_____________ residue in the protein. Ans: asparagine Section: 11.3

Chapter 11 Carbohydrates 心 8 When the carbohydrate portion is attached to a serine or threonine residue in a glycoprotein,it is referred to as a(n) glycoprotein. Ans:O-linked Section:11.3 9 The influenza virus recognizes residues of glycoproteins present on cell surface. Ans:sialic acid Section:11.4 10 In C-type lectins,a acts as a bridge between the carbohydrate and the protein Ans:calcium ion Section:11.4 Multiple Choice Questions 1 Carbohydrates are A)polyhydroxy aldehydes. B) polyhydroxy ketones. C) polyhydroxy acids. D) polyhydroxy alcohols. E) a and b. Ans:E Section:Introduction 2 The simplest carbohydrates are A) D-and L-glyceraldehyde. B) dihydroxyacetone and D-and L-glyceraldehyde. C) dihydroxyacetone and glycerate. D) All of the above. E) None of the above Ans:B Section:11.1 3 An aldehyde and alcohol can react to form a A) hemiaketal. D) All of the above. B) hemiketal E) None of the above. C) hemiacetal. Ans:C Section:11.1 4 Fructose can cyclize to (a) A) pyranose ring. B) furanose ring. C) both pyranose and furanose ring forms. D) All of the above. E)None of the above. Ans:C Section:11.1 5 The nutritional storage form(s)of glucose in plants. A)glycogen B)amylose C)amylopectin D)b and c E)All of the above Ans:D Section:11.2
Chapter 11 Carbohydrates 3 8 When the carbohydrate portion is attached to a serine or threonine residue in a glycoprotein, it is referred to as a(n)_______________ glycoprotein. Ans: O-linked Section: 11.3 9 The influenza virus recognizes _______________ residues of glycoproteins present on cell surface. Ans: sialic acid Section: 11.4 10 In C-type lectins, a _______________ acts as a bridge between the carbohydrate and the protein. Ans: calcium ion Section: 11.4 Multiple Choice Questions 1 Carbohydrates are A) polyhydroxy aldehydes. B) polyhydroxy ketones. C) polyhydroxy acids. D) polyhydroxy alcohols. E) a and b. Ans: E Section: Introduction 2 The simplest carbohydrates are A) D- and L-glyceraldehyde. B) dihydroxyacetone and D- and L-glyceraldehyde. C) dihydroxyacetone and glycerate. D) All of the above. E) None of the above. Ans: B Section: 11.1 3 An aldehyde and alcohol can react to form a A) hemiaketal. D) All of the above. B) hemiketal. E) None of the above. C) hemiacetal. Ans: C Section: 11.1 4 Fructose can cyclize to (a) A) pyranose ring. B) furanose ring. C) both pyranose and furanose ring forms. D) All of the above. E) None of the above. Ans: C Section: 11.1 5 The nutritional storage form(s) of glucose in plants. A) glycogen B) amylose C) amylopectin D) b and c E) All of the above Ans: D Section: 11.2
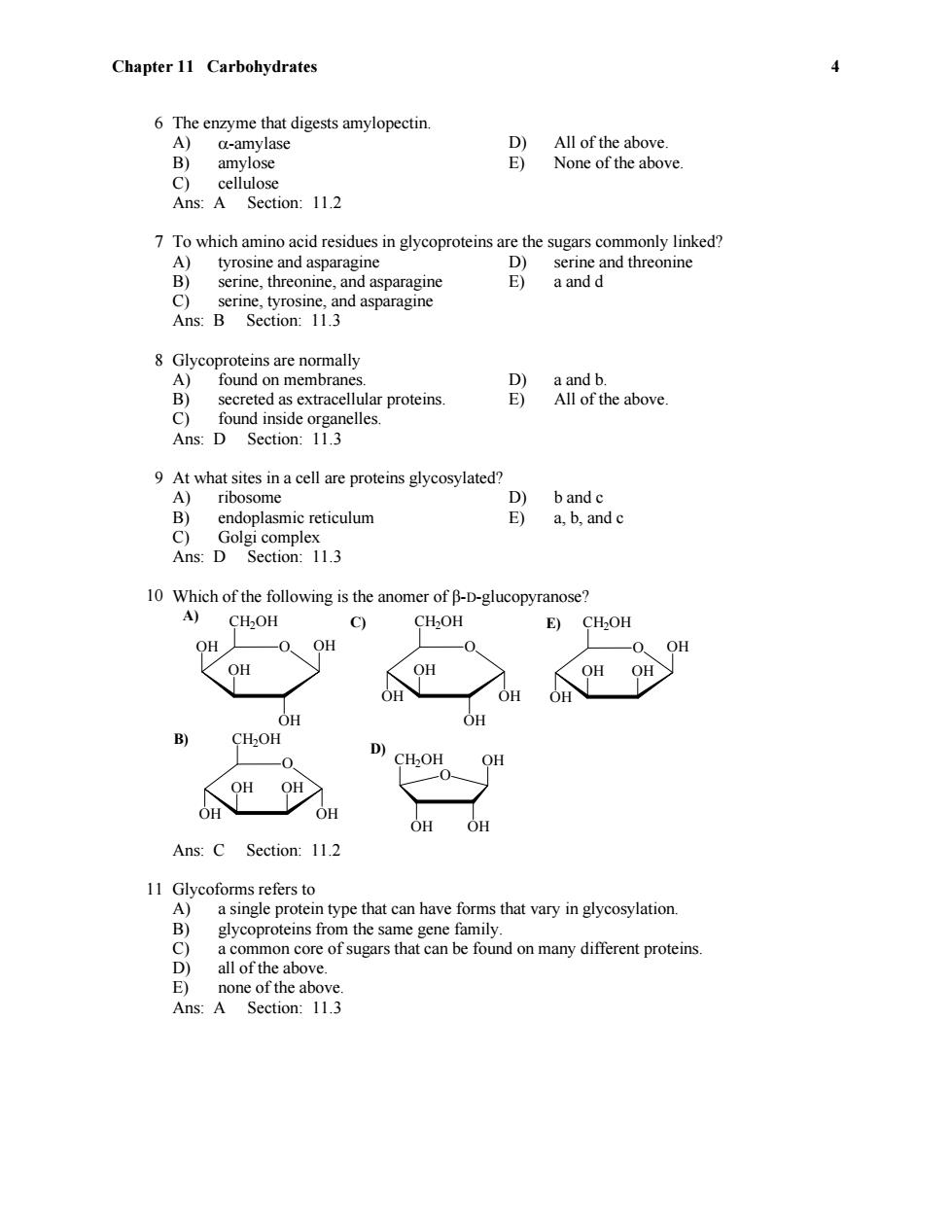
Chapter 11 Carbohydrates 4 6 The enzyme that digests amylopectin. A) a-amylase D) All of the above. B) amylose E) None of the above. C) cellulose Ans:A Section:11.2 7 To which amino acid residues in glycoproteins are the sugars commonly linked? A) tyrosine and asparagine D) serine and threonine B) serine,threonine,and asparagine E) a and d C)serine,tyrosine,and asparagine Ans:B Section:11.3 8 Glycoproteins are normally A)found on membranes. D) a and b. B) secreted as extracellular proteins E) All of the above. C) found inside organelles. Ans:D Section:11.3 9 At what sites in a cell are proteins glycosylated? A)ribosome D) b and c B) endoplasmic reticulum E) a,b,and c C) Golgi complex Ans:D Section:11.3 10 Which of the following is the anomer of B-D-glucopyranose? A) CH2OH c) CH2OH E) CH2OH OH 0. OH 0 -0 OH OH OH OH OH OH OH OH OH OH 乡 CH2OH D) 0 CHOH OH OH OH OH OH OH Ans:C Section:11.2 11 Glycoforms refers to A)a single protein type that can have forms that vary in glycosylation. B) glycoproteins from the same gene family. C) a common core of sugars that can be found on many different proteins. D) all of the above. E) none of the above. Ans:A Section:11.3
Chapter 11 Carbohydrates 4 6 The enzyme that digests amylopectin. A) -amylase D) All of the above. B) amylose E) None of the above. C) cellulose Ans: A Section: 11.2 7 To which amino acid residues in glycoproteins are the sugars commonly linked? A) tyrosine and asparagine D) serine and threonine B) serine, threonine, and asparagine E) a and d C) serine, tyrosine, and asparagine Ans: B Section: 11.3 8 Glycoproteins are normally A) found on membranes. D) a and b. B) secreted as extracellular proteins. E) All of the above. C) found inside organelles. Ans: D Section: 11.3 9 At what sites in a cell are proteins glycosylated? A) ribosome D) b and c B) endoplasmic reticulum E) a, b, and c C) Golgi complex Ans: D Section: 11.3 10 Which of the following is the anomer of β-D-glucopyranose? E) D) C) B) A) O OH OH O CH2OH OH OH CH2OH OH OH OH O OH CH2OH OH OH OH O OH CH2OH OH OH O OH OH OH CH2OH OH OH Ans: C Section: 11.2 11 Glycoforms refers to A) a single protein type that can have forms that vary in glycosylation. B) glycoproteins from the same gene family. C) a common core of sugars that can be found on many different proteins. D) all of the above. E) none of the above. Ans: A Section: 11.3

Chapter 11 Carbohydrates 12 Selectins are proteins that A) selectively bind proteins destined for lysozomes. B) aid in selection of proteins bound for the Golgi complex. c) bind immune-system cells as part of the inflammatory response. D) all of the above. E)none of the above. Ans:C Section:11.4 13 What are lectins? A) proteins that bind the carbohydrates on glycoproteins and other macromolecules B) proteins that promote cell-cell interaction C) proteins found in animals,plants,and microorganisms D) All of the above. E)None of the above. Ans:D Section:11.4. 14 How do some viruses gain entry into specific cells? A)by attaching to ion channels B) by cleaving the glycosidic bonds and altering protein shapes C) by binding to glycoproteins on the cell surface that are unique to specific cells D) All of the above. E) None of the above. Ans:C Section:11.4 15 Inhibitors against this viral enzyme have potential as anti-influenza agents. A) calnexin D) All of the above. B) neuramidase E) None of the above. C) selectin Ans:B Section:11.4 Short-Answer Questions 1 List some of the reasons carbohydrates are considered important molecules. Ans:Carbohydrates serve several important functions as fuels,metabolic intermediates,and energy stores.They are the basis of most of the organic matter on our planet.Carbohydrates serve as the structural framework or building blocks for DNA,RNA,and polysaccharides. They are also linked to other molecules,such as proteins and lipids,and play important roles in signaling and structure. Section:Introduction
Chapter 11 Carbohydrates 5 12 Selectins are proteins that A) selectively bind proteins destined for lysozomes. B) aid in selection of proteins bound for the Golgi complex. C) bind immune-system cells as part of the inflammatory response. D) all of the above. E) none of the above. Ans: C Section: 11.4 13 What are lectins? A) proteins that bind the carbohydrates on glycoproteins and other macromolecules B) proteins that promote cell-cell interaction C) proteins found in animals, plants, and microorganisms D) All of the above. E) None of the above. Ans: D Section: 11.4. 14 How do some viruses gain entry into specific cells? A) by attaching to ion channels B) by cleaving the glycosidic bonds and altering protein shapes C) by binding to glycoproteins on the cell surface that are unique to specific cells D) All of the above. E) None of the above. Ans: C Section: 11.4 15 Inhibitors against this viral enzyme have potential as anti-influenza agents. A) calnexin D) All of the above. B) neuramidase E) None of the above. C) selectin Ans: B Section: 11.4 Short-Answer Questions 1 List some of the reasons carbohydrates are considered important molecules. Ans: Carbohydrates serve several important functions as fuels, metabolic intermediates, and energy stores. They are the basis of most of the organic matter on our planet. Carbohydrates serve as the structural framework or building blocks for DNA, RNA, and polysaccharides. They are also linked to other molecules, such as proteins and lipids, and play important roles in signaling and structure. Section: Introduction
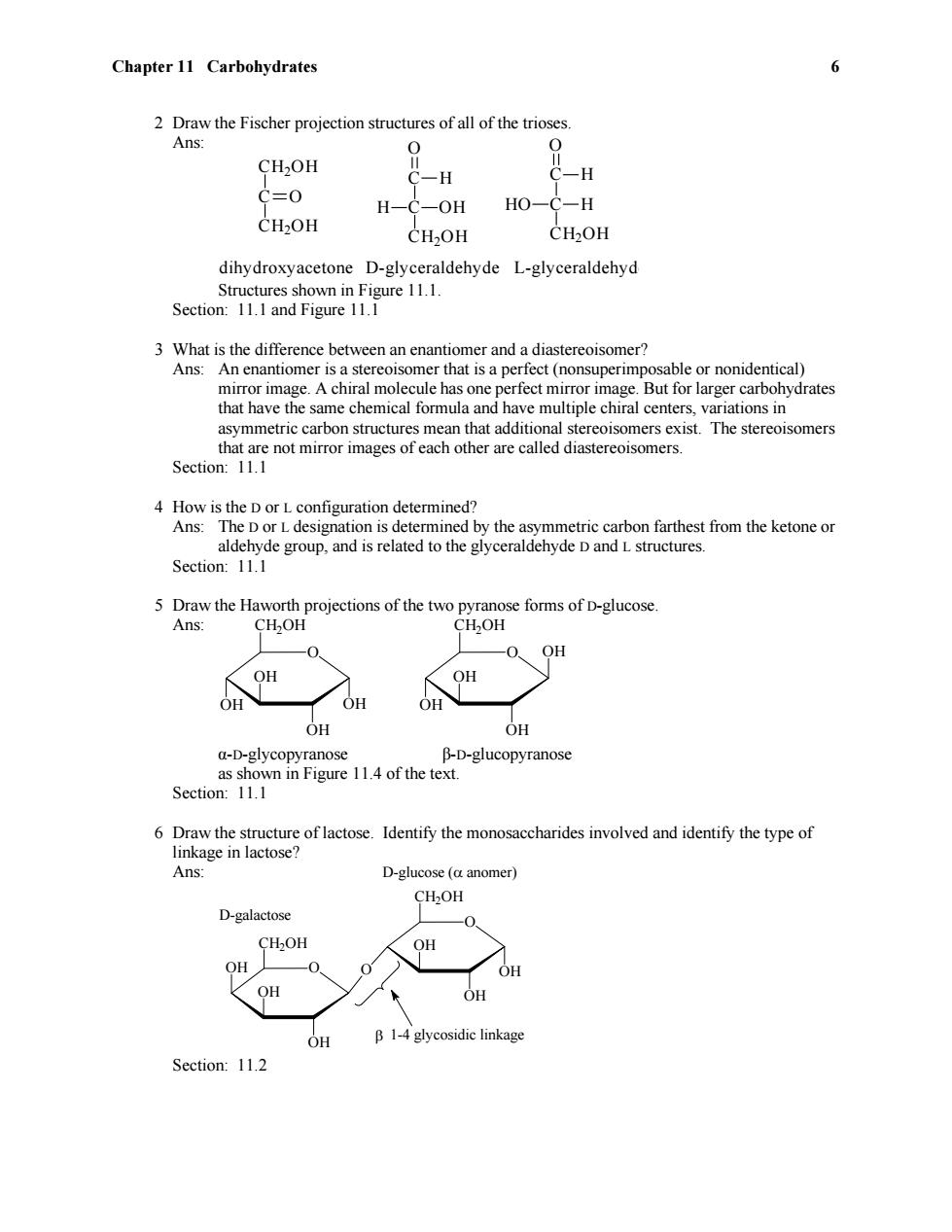
Chapter 11 Carbohydrates 6 2 Draw the Fischer projection structures of all of the trioses. Ans: 0 0 CH2OH C-H C -H C=0 H-C-OH HO-C-H CH2OH CH2OH CH2OH dihydroxyacetone D-glyceraldehyde L-glyceraldehyd Structures shown in Figure 11.1. Section:11.1 and Figure 11.1 3 What is the difference between an enantiomer and a diastereoisomer? Ans:An enantiomer is a stereoisomer that is a perfect(nonsuperimposable or nonidentical) mirror image.A chiral molecule has one perfect mirror image.But for larger carbohydrates that have the same chemical formula and have multiple chiral centers,variations in asymmetric carbon structures mean that additional stereoisomers exist.The stereoisomers that are not mirror images of each other are called diastereoisomers. Section:11.1 4 How is the D or L configuration determined? Ans:The D or L designation is determined by the asymmetric carbon farthest from the ketone or aldehyde group,and is related to the glyceraldehyde D and L structures. Section:11.1 5 Draw the Haworth projections of the two pyranose forms of D-glucose. Ans: CH2OH CH2OH OH OH OH OH OH OH OH a-D-glycopyranose B-D-glucopyranose as shown in Figure 11.4 of the text. Section:11.1 6 Draw the structure of lactose.Identify the monosaccharides involved and identify the type of linkage in lactose? Ans: D-glucose (a anomer) CH2OH D-galactose 0 CH2OH OH OH OH OH OH OH B 1-4 glycosidic linkage Section:11.2
Chapter 11 Carbohydrates 6 2 Draw the Fischer projection structures of all of the trioses. Ans: CH2OH C CH2OH O C C CH2OH O H H OH C C CH2OH O H HO H dihydroxyacetone D-glyceraldehyde L-glyceraldehyde Structures shown in Figure 11.1. Section: 11.1 and Figure 11.1 3 What is the difference between an enantiomer and a diastereoisomer? Ans: An enantiomer is a stereoisomer that is a perfect (nonsuperimposable or nonidentical) mirror image. A chiral molecule has one perfect mirror image. But for larger carbohydrates that have the same chemical formula and have multiple chiral centers, variations in asymmetric carbon structures mean that additional stereoisomers exist. The stereoisomers that are not mirror images of each other are called diastereoisomers. Section: 11.1 4 How is the D or L configuration determined? Ans: The D or L designation is determined by the asymmetric carbon farthest from the ketone or aldehyde group, and is related to the glyceraldehyde D and L structures. Section: 11.1 5 Draw the Haworth projections of the two pyranose forms of D-glucose. Ans: O OH OH CH2OH OH OH O OH OH CH2OH OH OH α-D-glycopyranose β-D-glucopyranose as shown in Figure 11.4 of the text. Section: 11.1 6 Draw the structure of lactose. Identify the monosaccharides involved and identify the type of linkage in lactose? Ans: O OH OH CH2OH OH O OH OH CH2OH O OH 1-4 glycosidic linkage D-glucose ( anomer) D-galactose Section: 11.2

Chapter 11 Carbohydrates 7 7 Compare the structures of amylopectin and amylose. Ans:Both are homopolymers of glucose.Amylose consists of unbranched a-1,4 linkages of glucose.Amylopectin is a branched structure,and contains both a-1,4 linkages and a-1,6 linkages,with the a-1,6 branches occurring about once every 30 glucose residues. Section:11.2 8 What are the chemical and structural differences between cellulose and glycogen? Ans:Both are glucose homopolymers.Glycogen is a branched polymer and contains a-1,4 linkages with B-1,6 branchpoints about every 10 residues.Cellulose is a linear polymer that contains B-1,4 linkages.Because of the B linkages,cellulose can form very long straight chains which can form interchain hydrogen bonds to form fibrils. Section:11.2 9 Describe some of the functions of glycosaminoglycans and proteoglycans. Ans: They function as lubricants,anticoagulents,and structural components;are important in pathways stimulating cell proliferation;and aid in mediating cell adhesion to extracellular matrices. Section:11.2 10 How does a genetic mutation account for some of the different human blood types? Ans:Blood type is determined by specific glycosyltransferases that add the end sugar to the glycoproteins found on red blood cells.Three different types of glycosyltransferase genes can be inherited,and each individual receives one from each parent.Two different forms result in the A and B blood types.A mutation in a third type results in a truncated product that is not active. Section:11.2 11 What is the advantage of having different blood types within a species? Ans:Variations are protective because differences may be critical to protection against disease and infection.A microorganism that gains advantage over a host by mimicking and/or using specific antigens,will not survive in host members that have differing antigens. Section:11.2 12 Describe the mechanism by which N-linked sugars are synthesized and attached to proteins. Ans:The units are assembled in the ER attached to dolichol phosphate scaffold.Two N-acetylglucosamine residues and five mannose residues are added to form a common precursor,which is flipped into the ER lumen.Then specific enzymes add dolichol-phosphate-linked sugars,forming a 14-residue core.This is transferred to an asparagine residue on a protein,and the sugar core is processed by removal of three glucose molecules.The addition and removal of other sugars in the Golgi complex further modify the carbohydrate. Section:11.3 and Figure 11.2 13 What are the two primary functions of the Golgi complex? Ans:The Golgi complex is 1)the site of carbohydrate addition and modification to glycoproteins,and 2)the major protein sorting center of the cell. Section:11.3
Chapter 11 Carbohydrates 7 7 Compare the structures of amylopectin and amylose. Ans: Both are homopolymers of glucose. Amylose consists of unbranched -1,4 linkages of glucose. Amylopectin is a branched structure, and contains both -1,4 linkages and -1,6 linkages, with the -1,6 branches occurring about once every 30 glucose residues. Section: 11.2 8 What are the chemical and structural differences between cellulose and glycogen? Ans: Both are glucose homopolymers. Glycogen is a branched polymer and contains -1,4 linkages with -1,6 branchpoints about every 10 residues. Cellulose is a linear polymer that contains -1,4 linkages. Because of the linkages, cellulose can form very long straight chains which can form interchain hydrogen bonds to form fibrils. Section: 11.2 9 Describe some of the functions of glycosaminoglycans and proteoglycans. Ans: They function as lubricants, anticoagulents, and structural components; are important in pathways stimulating cell proliferation; and aid in mediating cell adhesion to extracellular matrices. Section: 11.2 10 How does a genetic mutation account for some of the different human blood types? Ans: Blood type is determined by specific glycosyltransferases that add the end sugar to the glycoproteins found on red blood cells. Three different types of glycosyltransferase genes can be inherited, and each individual receives one from each parent. Two different forms result in the A and B blood types. A mutation in a third type results in a truncated product that is not active. Section: 11.2 11 What is the advantage of having different blood types within a species? Ans: Variations are protective because differences may be critical to protection against disease and infection. A microorganism that gains advantage over a host by mimicking and/or using specific antigens, will not survive in host members that have differing antigens. Section: 11.2 12 Describe the mechanism by which N-linked sugars are synthesized and attached to proteins. Ans: The units are assembled in the ER attached to dolichol phosphate scaffold. Two N-acetylglucosamine residues and five mannose residues are added to form a common precursor, which is flipped into the ER lumen. Then specific enzymes add dolichol-phosphatelinked sugars, forming a 14-residue core. This is transferred to an asparagine residue on a protein, and the sugar core is processed by removal of three glucose molecules. The addition and removal of other sugars in the Golgi complex further modify the carbohydrate. Section: 11.3 and Figure 11.2 13 What are the two primary functions of the Golgi complex? Ans: The Golgi complex is 1) the site of carbohydrate addition and modification to glycoproteins, and 2) the major protein sorting center of the cell. Section: 11.3

Chapter 11 Carbohydrates 8 14 What is the role of mannose 6-phosphate?What disease is caused by lack of this terminal sugar on glycoproteins? Ans:It acts as the marker that directs many lysozomal enzymes to their proper site.Without the modified mannose residue,the proteins are misdirected.For example,in I-cell disease, several enzymes are directed to blood and urine instead of the lysozomes.As a result, hydrolases required for glycosaminglycan and glycolipid degradation are missing,leading to deformity and retardation. Section:11.3 15 Why is it more difficult to determine the structure of the oligosaccharides,when compared to amino acid sequences? Ans:Amino acids are linked through peptide bonds and the side chains vary in size,charge,and chemical properties.In contrast,sugars can be branched,and can have a or B linkages, which makes determining the attachment difficult.Furthermore,many sugars have the same or similar chemical formula,and similar chemical properties,making specific identification and linkage difficult. Section:11.3
Chapter 11 Carbohydrates 8 14 What is the role of mannose 6-phosphate? What disease is caused by lack of this terminal sugar on glycoproteins? Ans: It acts as the marker that directs many lysozomal enzymes to their proper site. Without the modified mannose residue, the proteins are misdirected. For example, in I-cell disease, several enzymes are directed to blood and urine instead of the lysozomes. As a result, hydrolases required for glycosaminglycan and glycolipid degradation are missing, leading to deformity and retardation. Section: 11.3 15 Why is it more difficult to determine the structure of the oligosaccharides, when compared to amino acid sequences? Ans: Amino acids are linked through peptide bonds and the side chains vary in size, charge, and chemical properties. In contrast, sugars can be branched, and can have or linkages, which makes determining the attachment difficult. Furthermore, many sugars have the same or similar chemical formula, and similar chemical properties, making specific identification and linkage difficult. Section: 11.3