
上降充通大学 SHANGHAI JIAO TONG UNIVERSITY Chapter 8: Enzymes:Basic Concepts and Kinetics 酶:基本概念及动力学 Berg·Tymoczko·Stryer Biochemistry Sixth Edition
Berg • Tymoczko • Stryer Biochemistry Sixth Edition Chapter 8: Enzymes: Basic Concepts and Kinetics 酶:基本概念及动力学

OUTLINES 1.Enzymes Are Powerful and Highly Specific Catalysts一概论 2.Free Energy Is a Useful Thermodynamic Function for Understanding Enzymes-热力学 3.Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State一化学 4.The Michaelis-Menten Model Accounts for the Kinetic Properties of Many Enzymes一动力学 5.Enzymes Can Be Inhibited by Specific Molecules一抑制作用
1. Enzymes Are Powerful and Highly Specific Catalysts - 概论 2. Free Energy Is a Useful Thermodynamic Function for Understanding Enzymes -热力学 3. Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State -化学 4. The Michaelis-Menten Model Accounts for the Kinetic Properties of Many Enzymes -动力学 5. Enzymes Can Be Inhibited by Specific Molecules -抑制作用 OUTLINES

The most striking characteristics of enzymes: 一 Catalytic power(催化高效性) Specificity(选择性) Chemical nature: 一Proteins蛋白质(Nearly all known enzymes are proteins,but proteins are not an absolute monopoly on catalysis);
The most striking characteristics of enzymes: - Catalytic power (催化高效性) - Specificity (选择性) Chemical nature: - Proteins 蛋白质 (Nearly all known enzymes are proteins, but proteins are not an absolute monopoly on catalysis);
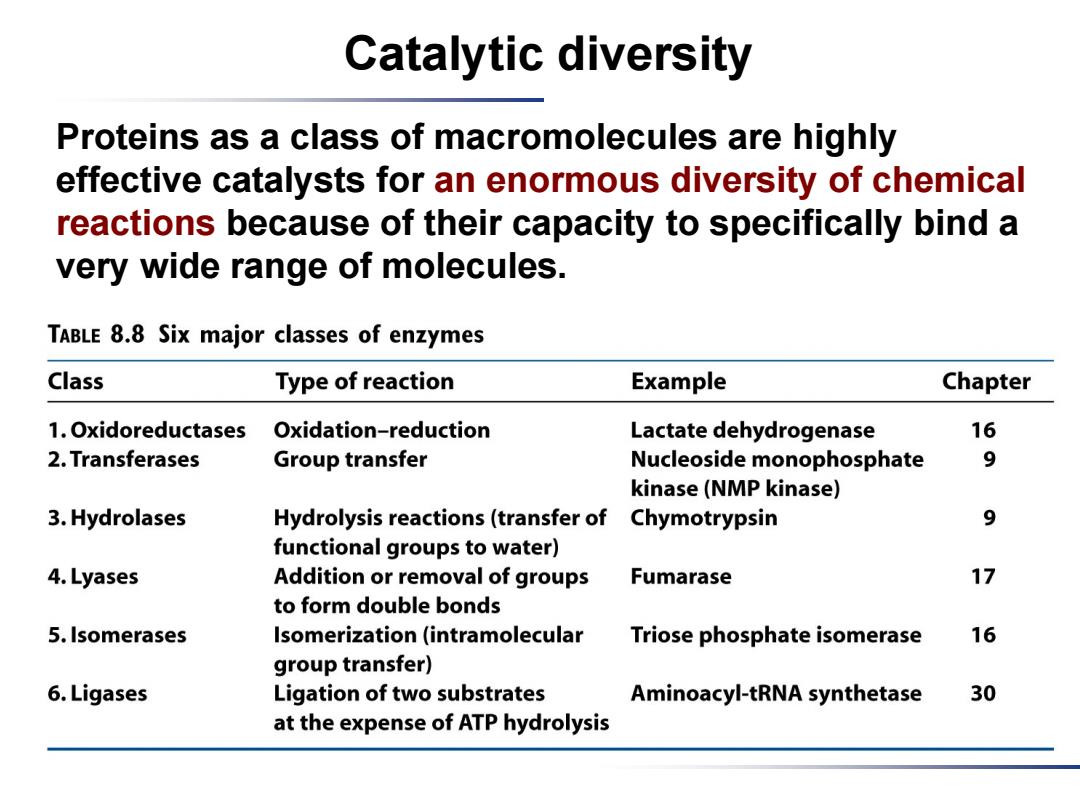
Catalytic diversity Proteins as a class of macromolecules are highly effective catalysts for an enormous diversity of chemical reactions because of their capacity to specifically bind a very wide range of molecules. TABLE 8.8 Six major classes of enzymes Class Type of reaction Example Chapter 1.Oxidoreductases Oxidation-reduction Lactate dehydrogenase 16 2.Transferases Group transfer Nucleoside monophosphate 9 kinase(NMP kinase) 3.Hydrolases Hydrolysis reactions(transfer of Chymotrypsin 9 functional groups to water) 4.Lyases Addition or removal of groups Fumarase 17 to form double bonds 5.Isomerases Isomerization(intramolecular Triose phosphate isomerase 16 group transfer) 6.Ligases Ligation of two substrates Aminoacyl-tRNA synthetase 30 at the expense of ATP hydrolysis
Catalytic diversity Proteins as a class of macromolecules are highly effective catalysts for an enormous diversity of chemical reactions because of their capacity to specifically bind a very wide range of molecules

Active site活性中心 Active site活性中心:Catalysis takes place at a particular site on the enzyme. Substrate ES complex Enryme By untilizing the full repertoire of intermolecular forces 分子间作用力,enzymes bring substrates together in an optimal orientation定位,the prelude to making and breaking chemical bonds.They catalyze reactions by stabilizing transition states:过渡态,the highest-energy speciesi高能物质in reaction pathways
Active site 活性中心:Catalysis takes place at a particular site on the enzyme. By untilizing the full repertoire of intermolecular forces 分子间作用力, enzymes bring substrates together in an optimal orientation定位, the prelude to making and breaking chemical bonds. They catalyze reactions by stabilizing transition states过渡态, the highest-energy species高能物质 in reaction pathways. Active site 活性中心

OUTLINES 1.Enzymes Are Powerful and Highly Specific Catalysts 2.Free Energy Is a Useful Thermodynamic Function for Understanding Enzymes-热力学 3.Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State一化学 4.The Michaelis-Menten Model Accounts for the Kinetic Properties of Many Enzymes一动力学 5.Enzymes Can Be Inhibited by Specific Molecules一抑制作用
1. Enzymes Are Powerful and Highly Specific Catalysts 2. Free Energy Is a Useful Thermodynamic Function for Understanding Enzymes -热力学 3. Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State -化学 4. The Michaelis-Menten Model Accounts for the Kinetic Properties of Many Enzymes -动力学 5. Enzymes Can Be Inhibited by Specific Molecules -抑制作用 OUTLINES
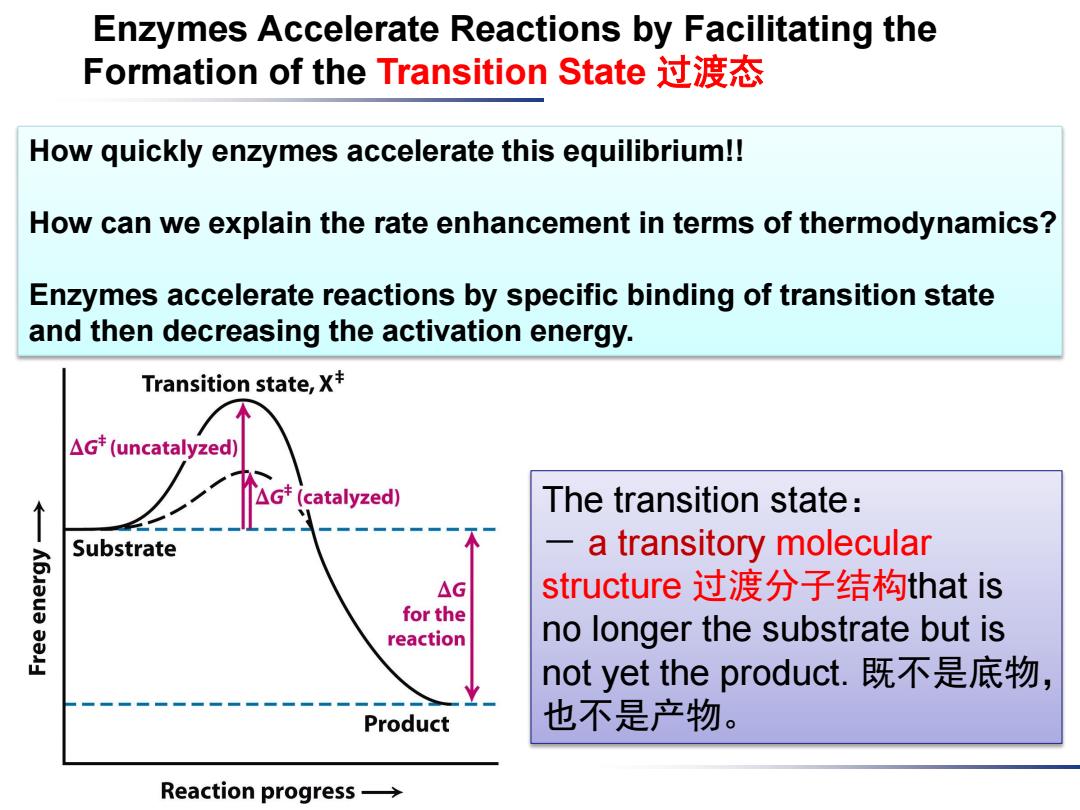
Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State过渡态 How quickly enzymes accelerate this equilibrium!! How can we explain the rate enhancement in terms of thermodynamics? Enzymes accelerate reactions by specific binding of transition state and then decreasing the activation energy. Transition state,X+ △Gt(uncatalyzed) The transition state: Substrate a transitory molecular △G structure过渡分子结构that is for the reaction no longer the substrate but is not yet the product.既不是底物, Product 也不是产物。 Reaction progress→
Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State 过渡态 The transition state: - a transitory molecular structure 过渡分子结构that is no longer the substrate but is not yet the product. 既不是底物, 也不是产物。 How quickly enzymes accelerate this equilibrium!! How can we explain the rate enhancement in terms of thermodynamics? Enzymes accelerate reactions by specific binding of transition state and then decreasing the activation energy

Transition State(过渡态) A chemical reaction of substrate s to form product P goes through a transition state S*that has a higher free energy than does either S or F.(高能过渡分子) The star denotes a thermodynamic property of the transition state.The transition state is the most seldom occupied species 很难捕捉along the reaction pathway because it is the one with the highest free energy.(不稳定) .The difference in free energy between the transition state and the substrate is called the Gibbs free energy of activation or simply the activation energy,symbolized by AG*. △G*=Gs*-Gs
Transition State (过渡态) • A chemical reaction of substrate S to form product P goes through a transition state S* that has a higher free energy than does either S or P. (高能过渡分子) • The star * denotes a thermodynamic property of the transition state. The transition state is the most seldom occupied species 很难捕捉 along the reaction pathway because it is the one with the highest free energy. (不稳定) •The difference in free energy between the transition state and the substrate is called the Gibbs free energy of activation or simply the activation energy, symbolized by ΔG*

Transition state,X* Note:AG*does not enter △Gt(uncatalyzed) into the final△G △G(catalyzed) calculation for the reaction, Substrate K6Jeua because the energy input △G for the required to reach the reaction transition state is returned when the transition state Product forms the product.△G*不 Reaction progress→ 参与到反应自由能,因为 形成时所需自由能和裂解 时释放自由能相抵消 Enzymes function:to lower the activation energy,or, in other words,enzymes facilitate the formation of the transition state
Enzymes function: to lower the activation energy, or, in other words, enzymes facilitate the formation of the transition state. Note: ΔG* does not enter into the final ΔG calculation for the reaction, because the energy input required to reach the transition state is returned when the transition state forms the product. ΔG*不 参与到反应自由能,因为 形成时所需自由能和裂解 时释放自由能相抵消

Assumption: the transition state (S*)and the substrate (S)are in equilibrium. in which K*is the equilibrium constant for the formation of S*,and v is the rate of formation of product from S*. The rate of the reaction is proportional to the concentration of S* Rate [S*] because only S*can be converted into product
Assumption: the transition state (S* ) and the substrate (S) are in equilibrium. in which K* is the equilibrium constant for the formation of S*, and v is the rate of formation of product from S*. The rate of the reaction is proportional to the concentration of S* Rate ∝ [S*] because only S* can be converted into product