
上降充通大学 SHANGHAI JIAO TONG UNIVERSITY Chapter 8: Enzymes:Basic Concepts and Kinetics 酶:基本概念及动力学 Berg·Tymoczko·Stryer Biochemistry Sixth Edition
Berg • Tymoczko • Stryer Biochemistry Sixth Edition Chapter 8: Enzymes: Basic Concepts and Kinetics 酶:基本概念及动力学

OUTLINES 1.Enzymes Are Powerful and Highly Specific Catalysts一概论 2.Free Energy Is a Useful Thermodynamic Function for Understanding Enzymes-热力学 3.Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State一化学 4.The Michaelis-Menten Model Accounts for the Kinetic Properties of Many Enzymes-动力学 5.Enzymes Can Be Inhibited by Specific Molecules一抑制作用
1. Enzymes Are Powerful and Highly Specific Catalysts - 概论 2. Free Energy Is a Useful Thermodynamic Function for Understanding Enzymes -热力学 3. Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State -化学 4. The Michaelis-Menten Model Accounts for the Kinetic Properties of Many Enzymes -动力学 5. Enzymes Can Be Inhibited by Specific Molecules -抑制作用 OUTLINES
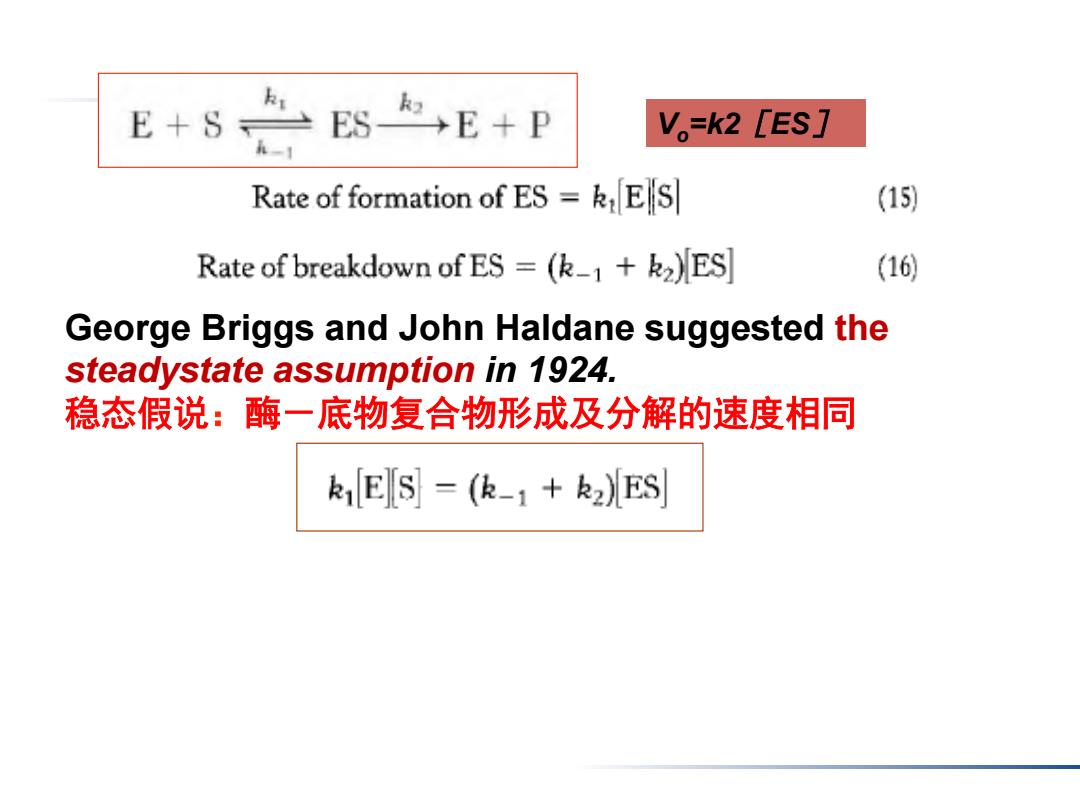
E+ESE+P V。=k2[ES] Rate of formation of ES=kES] (15) Rate of breakdown of ES =(k_1+k2)ES (16) George Briggs and John Haldane suggested the steadystate assumption in 1924. 稳态假说:酶一底物复合物形成及分解的速度相同 k1E S=(k-1+k2)ES
George Briggs and John Haldane suggested the steadystate assumption in 1924. 稳态假说:酶-底物复合物形成及分解的速度相同 Vo=k2[ES]
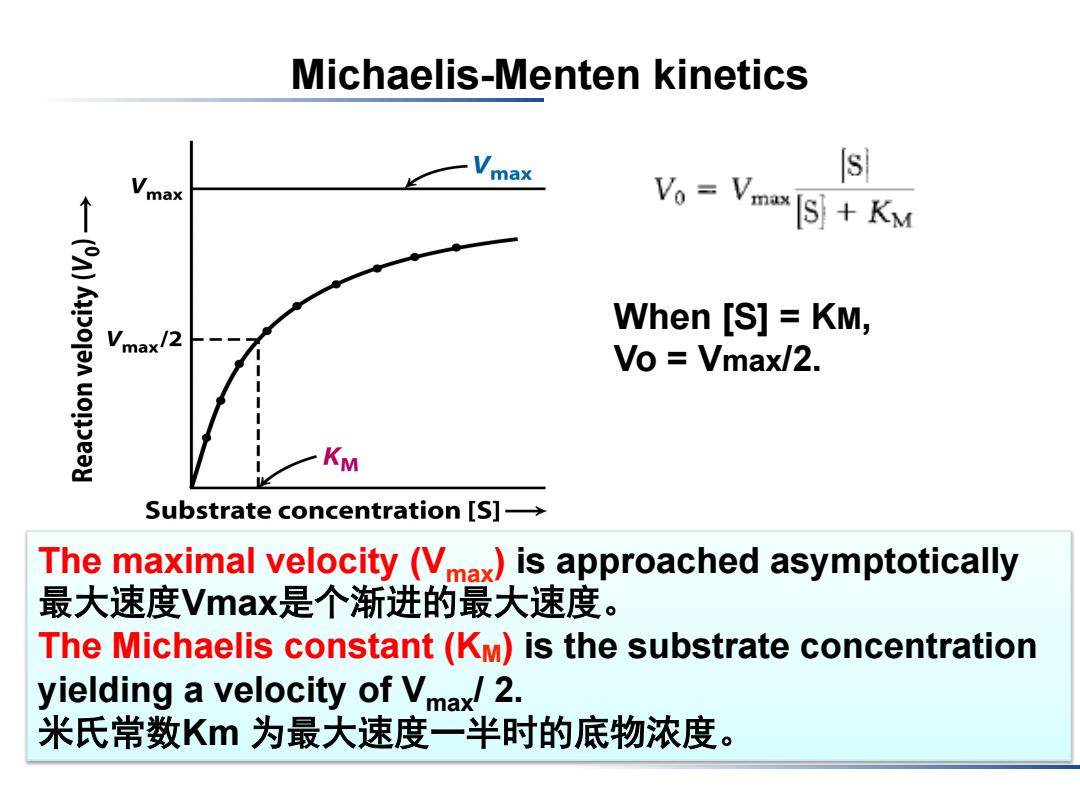
Michaelis-Menten kinetics max Vmax Vo Vmax IS KM When [S]KM, Vmax/2 Vo Vmax/2. KM Substrate concentration [S]- The maximal velocity (Vmax)is approached asymptotically 最大速度Vmax是个渐进的最大速度。 The Michaelis constant(KM)is the substrate concentration yielding a velocity of Vmax/2. 米氏常数Km为最大速度一半时的底物浓度
Michaelis-Menten kinetics The maximal velocity (Vmax) is approached asymptotically 最大速度Vmax是个渐进的最大速度。 The Michaelis constant (KM) is the substrate concentration yielding a velocity of Vmax/ 2. 米氏常数Km 为最大速度一半时的底物浓度。 When [S] = KM, Vo = Vmax/2
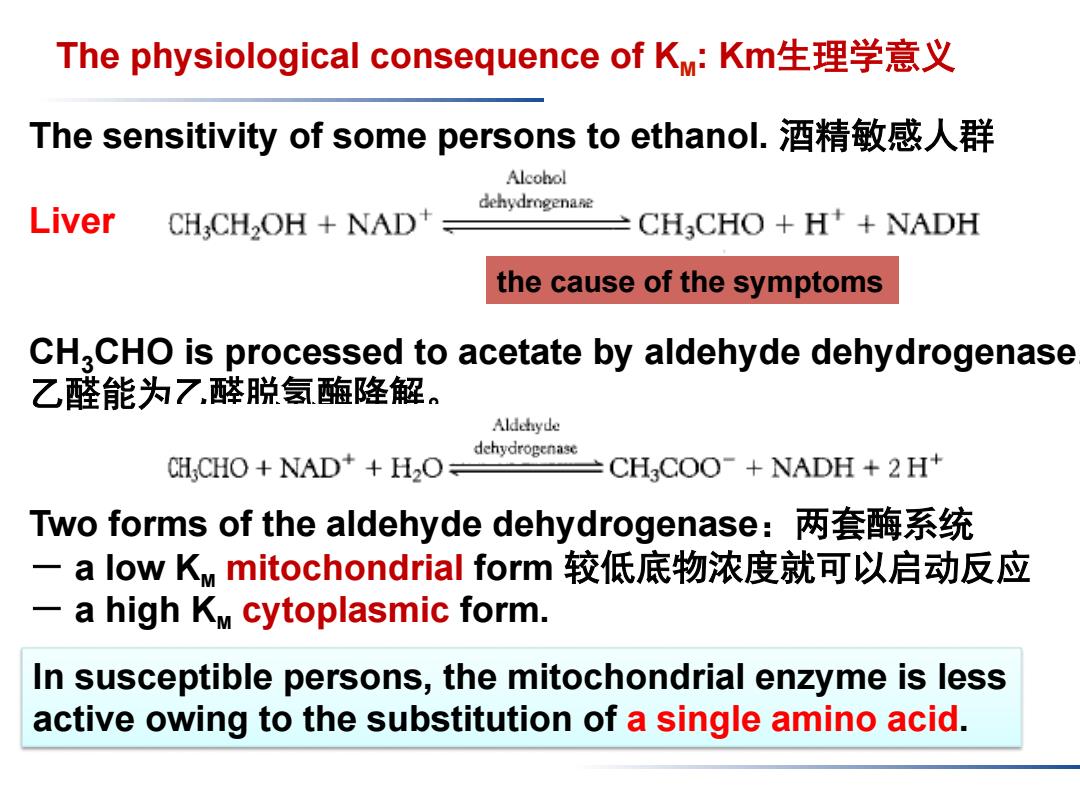
The physiological consequence of KM:Km生理学意义 The sensitivity of some persons to ethanol..酒精敏感人群 Alcohol dehydrogenane Liver CHCH2OH NAD CH CHO H+NADH the cause of the symptoms CH2CHO is processed to acetate by aldehyde dehydrogenase 乙醛能为7醛脱氢醢降解。 Aldehyde dehydrogenase CH;CHO NAD*+H2O= CH COO-NADH +2 H+ Two forms of the aldehyde dehydrogenase:两套酶系统 一a low KM mitochondrial form较低底物浓度就可以启动反应 -a high KM cytoplasmic form. In susceptible persons,the mitochondrial enzyme is less active owing to the substitution of a single amino acid
The sensitivity of some persons to ethanol. 酒精敏感人群 Liver CH3CHO is processed to acetate by aldehyde dehydrogenase. 乙醛能为乙醛脱氢酶降解。 Two forms of the aldehyde dehydrogenase:两套酶系统 - a low KM mitochondrial form 较低底物浓度就可以启动反应 - a high KM cytoplasmic form. The physiological consequence of KM: Km生理学意义 In susceptible persons, the mitochondrial enzyme is less active owing to the substitution of a single amino acid. the cause of the symptoms
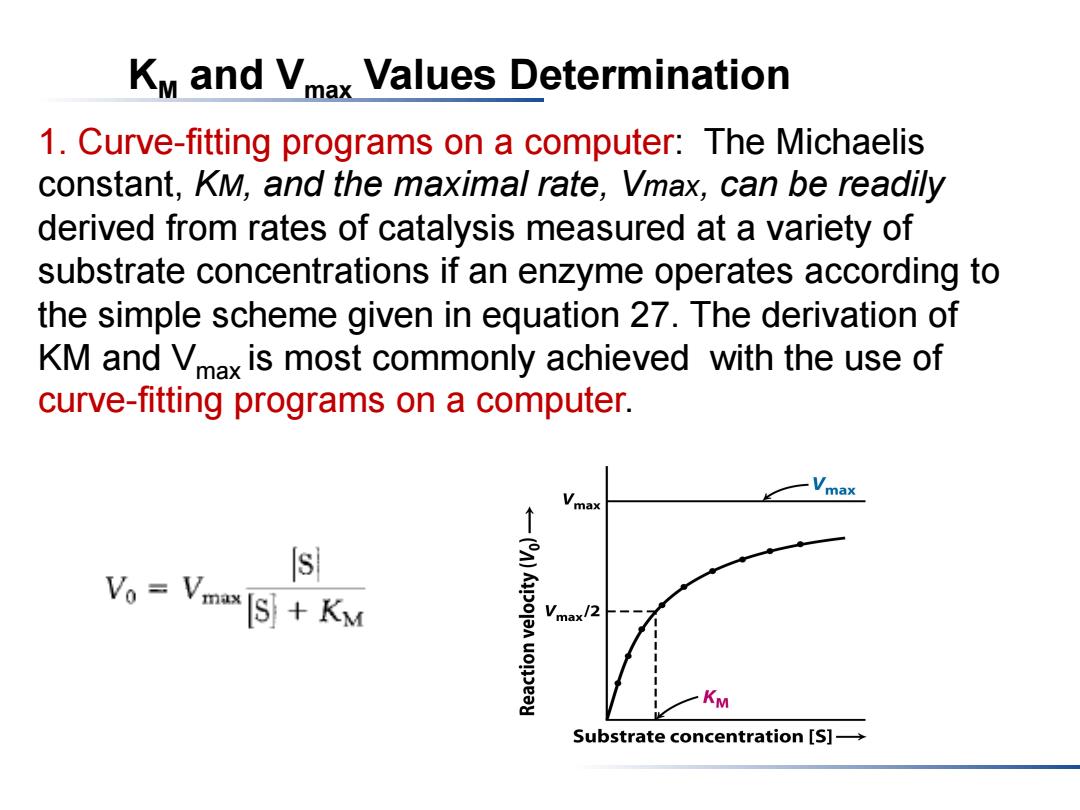
KM and Vmax Values Determination 1.Curve-fitting programs on a computer:The Michaelis constant,KM,and the maximal rate,Vmax,can be readily derived from rates of catalysis measured at a variety of substrate concentrations if an enzyme operates according to the simple scheme given in equation 27.The derivation of KM and Vmax is most commonly achieved with the use of curve-fitting programs on a computer. max s Vo Vmax [S]KM Vmax/2 KM Substrate concentration [S]-
KM and Vmax Values Determination 1. Curve-fitting programs on a computer: The Michaelis constant, KM, and the maximal rate, Vmax, can be readily derived from rates of catalysis measured at a variety of substrate concentrations if an enzyme operates according to the simple scheme given in equation 27. The derivation of KM and Vmax is most commonly achieved with the use of curve-fitting programs on a computer
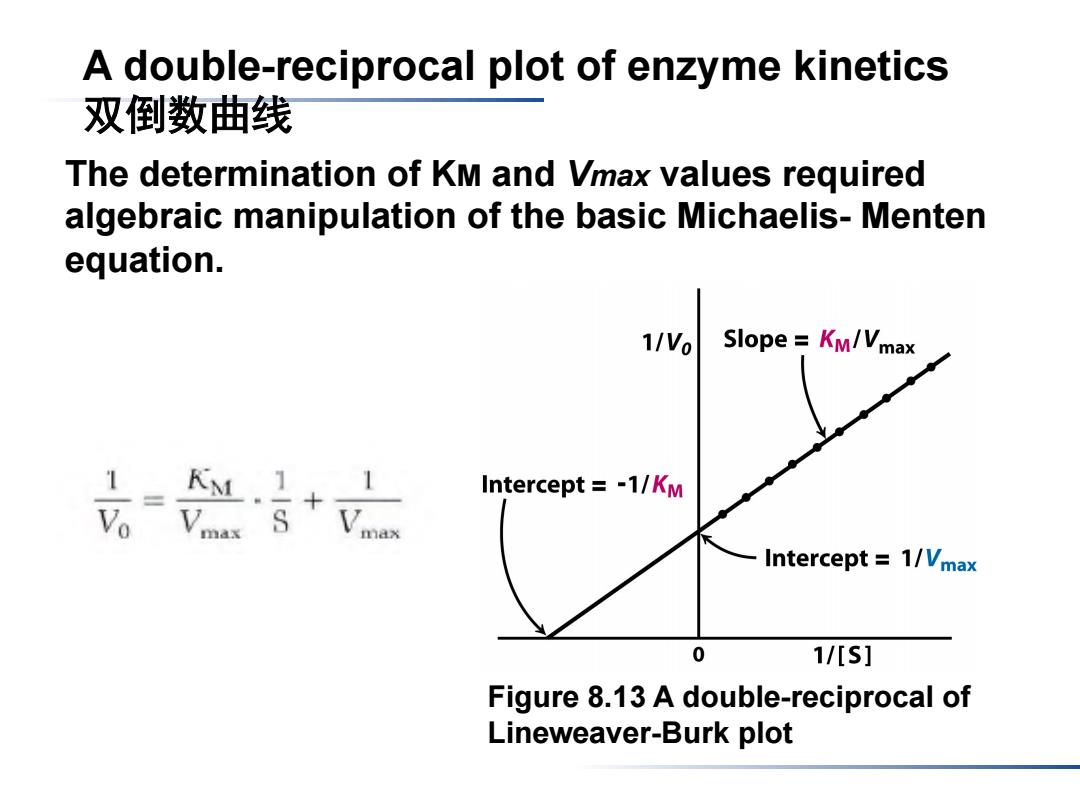
A double-reciprocal plot of enzyme kinetics 双倒数曲线 The determination of KM and Vmax values required algebraic manipulation of the basic Michaelis-Menten equation. 1/Vo Slope Km/Vmax 1 KM Intercept =-1/KM Intercept =1/Vmax 0 1/[s] Figure 8.13 A double-reciprocal of Lineweaver-Burk plot
Figure 8.13 A double-reciprocal of Lineweaver-Burk plot The determination of KM and Vmax values required algebraic manipulation of the basic Michaelis- Menten equation. A double-reciprocal plot of enzyme kinetics 双倒数曲线
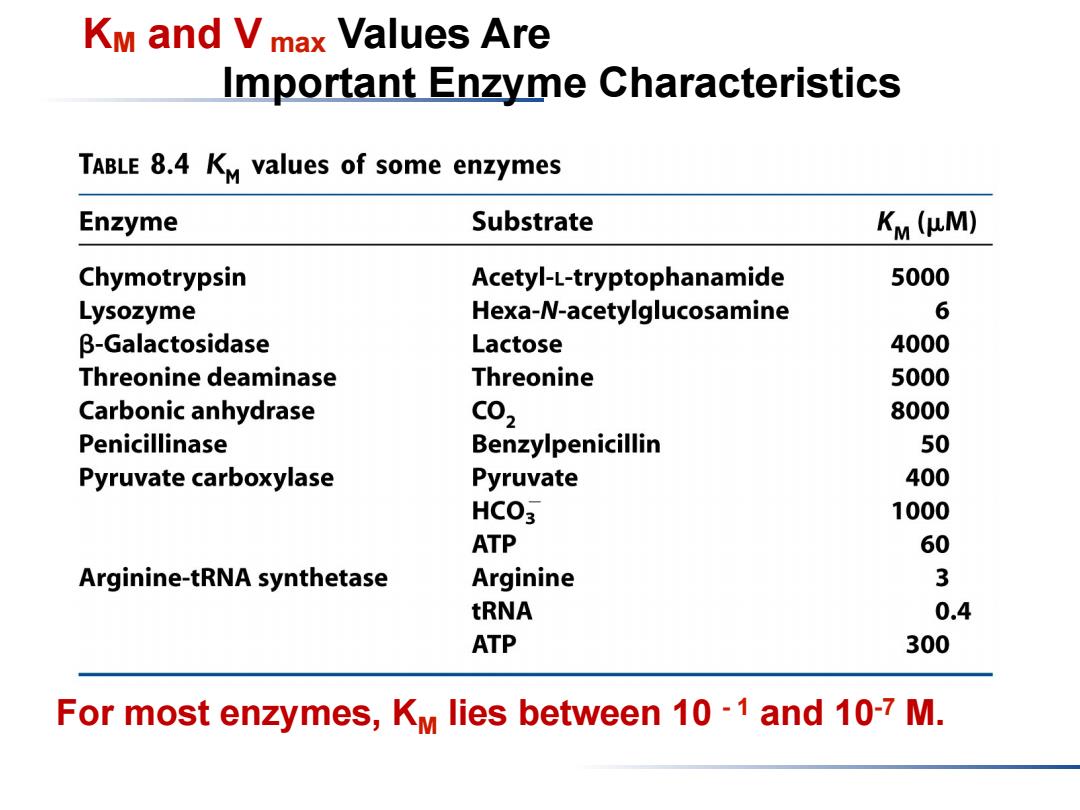
KM and Vmax Values Are Important Enzyme Characteristics TABLE 8.4 KM values of some enzymes Enzyme Substrate Km (HM) Chymotrypsin Acetyl-L-tryptophanamide 5000 Lysozyme Hexa-N-acetylglucosamine 6 β-Galactosidase Lactose 4000 Threonine deaminase Threonine 5000 Carbonic anhydrase C02 8000 Penicillinase Benzylpenicillin 50 Pyruvate carboxylase Pyruvate 400 HCO3 1000 ATP 60 Arginine-tRNA synthetase Arginine 3 tRNA 0.4 ATP 300 For most enzymes,KM lies between 10-1 and 10-7 M
KM and V max Values Are Important Enzyme Characteristics For most enzymes, KM lies between 10 - 1 and 10-7 M

Km is the concentration of substrate at which half the active sites are filled.Thus,Km provides a measure of the substrate concentration required for significant catalysis to take place. 因为KM值是酶活性部位填充一半时底物浓度,所以可以提 供了测定底物浓度的手段。 For many enzymes,experimental evidence suggests that Km provides an approximation of substrate concentration in vivo. 对很多酶来讲,KM提供了体内可能的底物浓度
KM is the concentration of substrate at which half the active sites are filled. Thus, KM provides a measure of the substrate concentration required for significant catalysis to take place. 因为KM值是酶活性部位填充一半时底物浓度,所以可以提 供了测定底物浓度的手段。 For many enzymes, experimental evidence suggests that KM provides an approximation of substrate concentration in vivo. 对很多酶来讲, KM 提供了体内可能的底物浓度
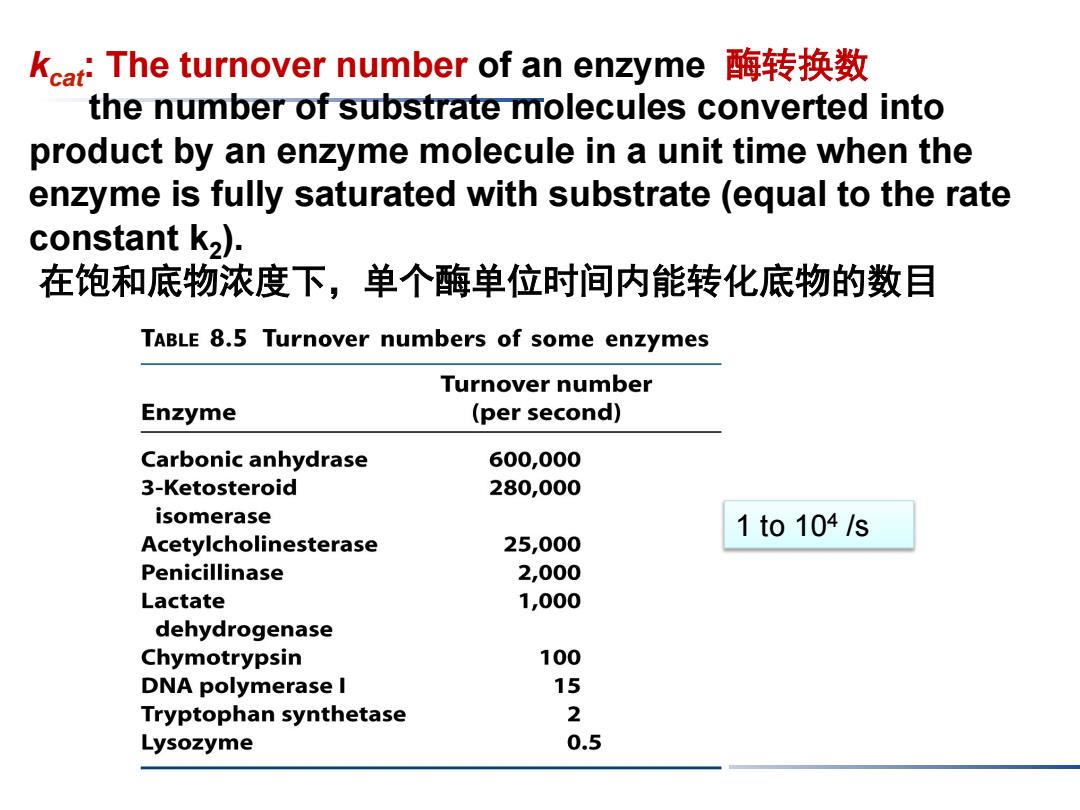
kcat:The turnover number of an enzyme酶转换数 the number of substrate molecules converted into product by an enzyme molecule in a unit time when the enzyme is fully saturated with substrate(equal to the rate constant k,). 在饱和底物浓度下,单个酶单位时间内能转化底物的数目 TABLE 8.5 Turnover numbers of some enzymes Turnover number Enzyme (per second) Carbonic anhydrase 600,000 3-Ketosteroid 280,000 isomerase 1to104/s Acetylcholinesterase 25,000 Penicillinase 2,000 Lactate 1,000 dehydrogenase Chymotrypsin 100 DNA polymerase I 15 Tryptophan synthetase 2 Lysozyme 0.5
1 to 104 /s kcat: The turnover number of an enzyme 酶转换数 the number of substrate molecules converted into product by an enzyme molecule in a unit time when the enzyme is fully saturated with substrate (equal to the rate constant k2). 在饱和底物浓度下,单个酶单位时间内能转化底物的数目