
Chapter 1 Biochemistry:An Evolving Science Matching Questions Use the following to answer questions 1-10: Choose the correct answer from the list below.Not all of the answers will be used. a)uridine b)true c)AG d)thymine e)AH f)sugar-phosphate units g)covalent h)Archaea i)entropy j)system k)3 1)2 m)false 1.DNA is made from the building blocks adenine,guanine,cytosine,and Ans:d Section:1.2 2.The DNA backbone is made from repeating Ans:f Section:1.2 The number of hydrogen bonds formed between A and T. Ans:I Section:1.2 4. The number of hydrogen bonds formed between G and C. Ans:k Section:1.2 5.The fundamental groups of organisms include Eukarya,Bacteria,and Ans:h Section:1.1
Chapter 1 Biochemistry: An Evolving Science Matching Questions Use the following to answer questions 1-10: Choose the correct answer from the list below. Not all of the answers will be used. a) uridine b) true c) G d) thymine e) H f) sugar-phosphate units g) covalent h) Archaea i) entropy j) system k) 3 l) 2 m) false 1. DNA is made from the building blocks adenine, guanine, cytosine, and ____________. Ans: d Section: 1.2 2. The DNA backbone is made from repeating ____________. Ans: f Section: 1.2 3. ____________ The number of hydrogen bonds formed between A and T. Ans: l Section: 1.2 4. ____________ The number of hydrogen bonds formed between G and C. Ans: k Section: 1.2 5. The fundamental groups of organisms include Eukarya, Bacteria, and ____________. Ans: h Section: 1.1

Chapter 1 Biochemistry:An Evolving Science 2 6 The strongest bonds in molecules: Ans:g Section:1.3 7 Hydrogen bonds are usually stronger than covalent bonds(true/false). Ans:b Section:1.3 8 Matter within a defined region of space Ans:j Section:1.3 9 For spontaneous reactions the AG must be positive. Ans:m Section:1.3 10. Gibbs free energy. Ans:c Section:1.3 Multiple Choice Questions 11.The structure of DNA described by Watson and Crick included A) a double helix. B) the sugar phosphate backbone aligned in the center of the helix. C) the base pairs that are stacked on the inside of the double helix. D) a and b. E)a and c. Ans:E Section:1.2 12.What did Watson and Crick suggest to be significant about the base pairing found in the helix? A)It allowed the DNA to twist in a helix. B) The DNA could be circular. C It was a mechanism for copying. D) All of the above. E)None of the above. Ans:C Section:1.3
Chapter 1 Biochemistry: An Evolving Science 2 6. ____________ The strongest bonds in molecules: Ans: g Section: 1.3 7. ____________ Hydrogen bonds are usually stronger than covalent bonds (true/false). Ans: b Section: 1.3 8. ____________ Matter within a defined region of space. Ans: j Section: 1.3 9. ____________ For spontaneous reactions the G must be positive. Ans: m Section: 1.3 10. ____________ Gibbs free energy. Ans: c Section: 1.3 Multiple Choice Questions 11. The structure of DNA described by Watson and Crick included A) a double helix. B) the sugar phosphate backbone aligned in the center of the helix. C) the base pairs that are stacked on the inside of the double helix. D) a and b. E) a and c. Ans: E Section: 1.2 12. What did Watson and Crick suggest to be significant about the base pairing found in the helix? A) It allowed the DNA to twist in a helix. B) The DNA could be circular. C) It was a mechanism for copying. D) All of the above. E) None of the above. Ans: C Section: 1.3

Chapter 1 Biochemistry:An Evolving Science 13.Approximately what percentage of the human genome encodes for proteins? A) 50% B) 90% c) 10% D 3% E)None of the above Ans:D Section 1.4 14.What gives proteins such a dominant role in biochemistry? A)the variation in protein sizes B) the ability to act as a blueprint C)their ability to self-replicate D)their ability to spontaneously fold into complex three-dimensional structures E)All of the above. Ans:D Section:1.4 15.If the whole chain is used in a non-overlapping frame,how many amino acids are defined by this DNA sequence:ATGTTTGGACTA? A)four B)two C)twelve D)six E)three Ans:A Section:1.4 16.What is the H+]concentration in a urine sample that has a pH of 6? A)10-6M B) 10-8M C) 10°M D)10-14M E)8M Ans:B Section 1.3 17.Which of the following is considered as a noncovalent bond? A) electrostatic interactions D) All of the above. B) hydrogen bonds E) None of the above. C) van der Waals interactions Ans:D Section:1.3 18.The energies for hydrogen bonds are approximately A)400 kJ/mol. D 200 kJ/mol. B)100-240kJ/mol E) None of the above C)4-20 kJ/mol. Ans:C Section:1.3 19.What pairs of atoms in bases are involved in hydrogen bonds? A)N-H and O-H D) All of the above. B)N-H and S-H E) None of the above. C)O-H and P-O Ans:A Section:1.3
Chapter 1 Biochemistry: An Evolving Science 3 13. Approximately what percentage of the human genome encodes for proteins? A) 50% B) 90% C) 10% D) 3% E) None of the above. Ans: D Section 1.4 14. What gives proteins such a dominant role in biochemistry? A) the variation in protein sizes B) the ability to act as a blueprint C) their ability to self-replicate D) their ability to spontaneously fold into complex three-dimensional structures E) All of the above. Ans: D Section: 1.4 15. If the whole chain is used in a non-overlapping frame, how many amino acids are defined by this DNA sequence: ATGTTTGGACTA? A) four B) two C) twelve D) six E) three Ans: A Section: 1.4 16. What is the [H+ ] concentration in a urine sample that has a pH of 6? A) 10 -6 M B) 10 -8 M C) 10 6 M D) 10 -14 M E) 8 M Ans: B Section 1.3 17. Which of the following is considered as a noncovalent bond? A) electrostatic interactions D) All of the above. B) hydrogen bonds E) None of the above. C) van der Waals interactions Ans: D Section: 1.3 18. The energies for hydrogen bonds are approximately A) 400 kJ/mol. D) 200 kJ/mol. B) 100–240 kJ/mol. E) None of the above. C) 4–20 kJ/mol. Ans: C Section: 1.3 19. What pairs of atoms in bases are involved in hydrogen bonds? A) N—H and O—H D) All of the above. B) N—H and S—H E) None of the above. C) O—H and P—O Ans: A Section: 1.3

Chapter 1 Biochemistry:An Evolving Science 4 20.Typical van der Waals energies are about A) 4-20 kJ/mol D) All of the above. B) 2-4 kJ/mol. E) None of the above. C)200 kJ/mol. Ans:B Section:1.3 21.What two properties of water are important for biological interactions? A)the polarity of water D) a and c B)the density of water E) b and c C)the cohesive properties of water Ans:D Section:1.3 22.The First Law of Thermodynamics states A)diversity is the result of gradual evolution. B)the total entropy of a system and its surroundings always increases for a spontaneous process. C) the total energy of a system and its surroundings is constant. D) light is both particle and wave. E)None of the above. Ans:C Section:1.3 23.The Second Law of Thermodynamics states A)the total entropy of a system and its surroundings always increases for a spontaneous process. B) temperatures will always decrease. C) the total energy of a system and its surroundings is constant. D) diversity is the result of gradual evolution. E) None of the above. Ans:A Section:1.3 24.List atoms commonly found in biological molecules that are often hydrogen-bond acceptors. A)carbon B)oxygen C)nitrogen D)b and c E)All of the above. Ans:D Section:1.3 25.Enthalpy is defined as A)a spontaneous reaction. D) All of the above. B)the entropy of the system. E) None of the above. C)the heat content of a system. Ans:C Section:1.3 26.If a particular reaction has a negative AG,is it likely to occur? A)Not unless energy is added to the system. B)Yes,if it is coupled to another reaction. C)Yes,it is spontaneous. D)No,it will never occur. E)Yes,if it takes place within a constrained area. Ans:C Section:1.3
Chapter 1 Biochemistry: An Evolving Science 4 20. Typical van der Waals energies are about A) 4–20 kJ/mol. D) All of the above. B) 2–4 kJ/mol. E) None of the above. C) 200 kJ/mol. Ans: B Section: 1.3 21. What two properties of water are important for biological interactions? A) the polarity of water D) a and c B) the density of water E) b and c C) the cohesive properties of water Ans: D Section: 1.3 22. The First Law of Thermodynamics states A) diversity is the result of gradual evolution. B) the total entropy of a system and its surroundings always increases for a spontaneous process. C) the total energy of a system and its surroundings is constant. D) light is both particle and wave. E) None of the above. Ans: C Section: 1.3 23. The Second Law of Thermodynamics states A) the total entropy of a system and its surroundings always increases for a spontaneous process. B) temperatures will always decrease. C) the total energy of a system and its surroundings is constant. D) diversity is the result of gradual evolution. E) None of the above. Ans: A Section: 1.3 24. List atoms commonly found in biological molecules that are often hydrogen-bond acceptors. A) carbon B) oxygen C) nitrogen D) b and c E) All of the above. Ans: D Section: 1.3 25. Enthalpy is defined as A) a spontaneous reaction. D) All of the above. B) the entropy of the system. E) None of the above. C) the heat content of a system. Ans: C Section: 1.3 26. If a particular reaction has a negative G, is it likely to occur? A) Not unless energy is added to the system. B) Yes, if it is coupled to another reaction. C) Yes, it is spontaneous. D) No, it will never occur. E) Yes, if it takes place within a constrained area. Ans: C Section: 1.3
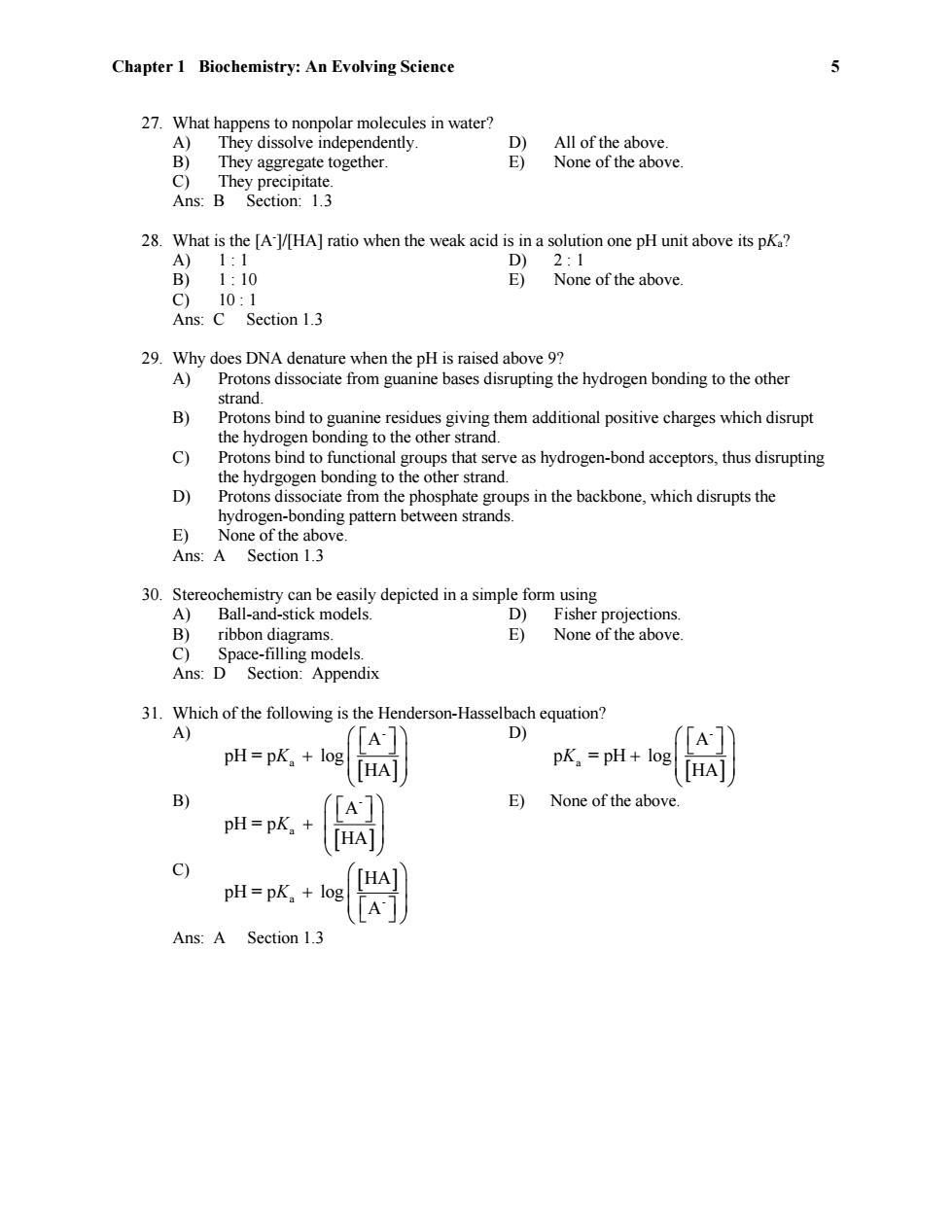
Chapter 1 Biochemistry:An Evolving Science 5 27.What happens to nonpolar molecules in water? A) They dissolve independently. D) All of the above. B) They aggregate together. E) None of the above. C)They precipitate. Ans:B Section:1.3 28.What is the [A-l/HA]ratio when the weak acid is in a solution one pH unit above its pKa? A)1:1 D) 2:1 B)1:10 E) None of the above. C)10:1 Ans:C Section 1.3 29.Why does DNA denature when the pH is raised above 9? A)Protons dissociate from guanine bases disrupting the hydrogen bonding to the other strand. B) Protons bind to guanine residues giving them additional positive charges which disrupt the hydrogen bonding to the other strand. c) Protons bind to functional groups that serve as hydrogen-bond acceptors,thus disrupting the hydrgogen bonding to the other strand. D) Protons dissociate from the phosphate groups in the backbone,which disrupts the hydrogen-bonding pattern between strands. E)None of the above. Ans:A Section 1.3 30.Stereochemistry can be easily depicted in a simple form using A) Ball-and-stick models. D)Fisher projections. B)ribbon diagrams. E)None of the above. C) Space-filling models. Ans:D Section:Appendix 31.Which of the following is the Henderson-Hasselbach equation? A) D) A pH=pK log pK。=pH+log HA HA B) E) None of the above. pH=pK. pH=pK。+ Ans:A Section 1.3
Chapter 1 Biochemistry: An Evolving Science 5 27. What happens to nonpolar molecules in water? A) They dissolve independently. D) All of the above. B) They aggregate together. E) None of the above. C) They precipitate. Ans: B Section: 1.3 28. What is the [A-]/[HA] ratio when the weak acid is in a solution one pH unit above its pKa? A) 1 : 1 D) 2 : 1 B) 1 : 10 E) None of the above. C) 10 : 1 Ans: C Section 1.3 29. Why does DNA denature when the pH is raised above 9? A) Protons dissociate from guanine bases disrupting the hydrogen bonding to the other strand. B) Protons bind to guanine residues giving them additional positive charges which disrupt the hydrogen bonding to the other strand. C) Protons bind to functional groups that serve as hydrogen-bond acceptors, thus disrupting the hydrgogen bonding to the other strand. D) Protons dissociate from the phosphate groups in the backbone, which disrupts the hydrogen-bonding pattern between strands. E) None of the above. Ans: A Section 1.3 30. Stereochemistry can be easily depicted in a simple form using A) Ball-and-stick models. D) Fisher projections. B) ribbon diagrams. E) None of the above. C) Space-filling models. Ans: D Section: Appendix 31. Which of the following is the Henderson-Hasselbach equation? A) - a A pH = p log HA K D) - a A p = pH log HA K B) - a A pH = p HA K E) None of the above. C) a - HA pH = p log A K Ans: A Section 1.3

Chapter 1 Biochemistry:An Evolving Science 6 32.What are the primary chemical components present in a phosphate buffer at pH 7.4? A)H3PO4 and PO43 D)H2PO4 and HPO42 B) H2PO4 and PO43 E)H3PO4 and HPO2 C)HPO42 and PO3 Ans:D Section 1.3 Short-Answer Questions 33.What are some of the medical implications of the human genome project? Ans:The obvious use is in diagnosing disease and in developing methods to treat and cure diseases.Physicians will be able to account for individual genetic differences in determining the best medical treatment. Section:Introduction 34.What is the significance of hydrogen bonding in biochemical structures such as DNA? Ans:The bonds are weak enough to be easily disrupted;yet when many are present,they provide the stabilization necessary for larger structures such as DNA. Section:1.2 35.What adaptation affected evolutionary diversity? Ans:Alteration of biochemical molecules and components to new roles is key to diversity and evolution Section:1.1 36.Describe resonance structures. Ans:Resonance structures are ways of writing covalent bonds in which two or more alternate bonding patterns can be achieved.This is due to the sharing of electrons over several atoms.Common examples are found in peptide bonds,and in some of the bases.Benzene is shown in the text. Section:1.3 37.What is an electrostatic interaction?Give an example. Ans:It is the attractive force of two oppositely charged atoms.Salts(such as NaCl)are a common example. Section:1.3 38.How is water able to be a solvent for so many biological molecules? Ans:Many biological molecules have polar characteristics.Water is extremely polar and is capable of competing with other polar molecules by weakening their electrostatic and hydrogen bonds.The oxygen can act as a hydrogen-bond acceptor,and the hydrogen can act as a donor. Section:1.3 39.What is the net effect of many van der Waals interactions? Ans:At the interface of two large molecules,the numerous van der Waals interactions can substantially affect and stabilize the interaction. Section:1.3
Chapter 1 Biochemistry: An Evolving Science 6 32. What are the primary chemical components present in a phosphate buffer at pH 7.4? A) H3PO4 and PO4 -3 D) H2PO4 - and HPO4 -2 B) H2PO4 - and PO4 -3 E) H3PO4 and HPO4 -2 C) HPO4 -2 and PO4 -3 Ans: D Section 1.3 Short-Answer Questions 33. What are some of the medical implications of the human genome project? Ans: The obvious use is in diagnosing disease and in developing methods to treat and cure diseases. Physicians will be able to account for individual genetic differences in determining the best medical treatment. Section: Introduction 34. What is the significance of hydrogen bonding in biochemical structures such as DNA? Ans: The bonds are weak enough to be easily disrupted; yet when many are present, they provide the stabilization necessary for larger structures such as DNA. Section: 1.2 35. What adaptation affected evolutionary diversity? Ans: Alteration of biochemical molecules and components to new roles is key to diversity and evolution. Section: 1.1 36. Describe resonance structures. Ans: Resonance structures are ways of writing covalent bonds in which two or more alternate bonding patterns can be achieved. This is due to the sharing of electrons over several atoms. Common examples are found in peptide bonds, and in some of the bases. Benzene is shown in the text. Section: 1.3 37. What is an electrostatic interaction? Give an example. Ans: It is the attractive force of two oppositely charged atoms. Salts (such as NaCl) are a common example. Section: 1.3 38. How is water able to be a solvent for so many biological molecules? Ans: Many biological molecules have polar characteristics. Water is extremely polar and is capable of competing with other polar molecules by weakening their electrostatic and hydrogen bonds. The oxygen can act as a hydrogen-bond acceptor, and the hydrogen can act as a donor. Section: 1.3 39. What is the net effect of many van der Waals interactions? Ans: At the interface of two large molecules, the numerous van der Waals interactions can substantially affect and stabilize the interaction. Section: 1.3

Chapter 1 Biochemistry:An Evolving Science 7 40.If most proteins are found surrounded by water in the cell,what type of functional groups would you expect to find on the surface of a water soluble protein? Ans:Polar and charged amino-acid residues would be present on the surface of the protein. Section:1.3 41.How are electrostatic forces used in protein folding? Ans:The attraction of two oppositely charged functional groups would be one of the forces helping to form the three-dimensional shape of the protein. Section:1.3 42.If the First Law of Thermodynamics is true,how can biological processes be carried out? Ans:Although energy cannot be created or destroyed,it can take on different forms,such as heat or chemical energy.Thus,the energy can be stored as chemical bond energy,which can be used to do work. Section:1.3 43.How can a cell exist if the Second Law of Thermodynamics is true? Ans:Entropy in a local area can be decreased,but only at the expense of increased entropy in the larger area,or universe. Section:1.3 44.Provide a simple example of entropy processes. Ans:Several examples can be provided,including the random mixture of atoms when two different gases are mixed,or the creation of water molecules from energy gained following the mixture of oxygen and hydrogen under certain conditions. Section:1.3 45.What does this equation mean: AG=△I system-T△S system<0? Ans:The free energy change(AG)must be negative for a reaction to be spontaneous.Only under these conditions can the total entropy increase. Section:1.3 46.What is the significance of using AG in biochemistry? Ans:Gibbs-free energy,also called the free-energy change,is used to describe the energetics of a reaction.This symbol is used to determine if particular reactions will be spontaneous or biologically feasible. Section:1.3 47.What thermodynamic and free-energy changes participate in protein folding? Ans:A combination of hydrogen bonds and van der Waals forces affect enthalpy and the entropy associated with hydrophobic interactions. Section:1.3 48.How do hydrophobic interactions aid in protein folding? Ans:Hydrophobic interaction causes some nonpolar amino acids to aggregate and form the interior of the protein.This results in a net release of heat and a favorable change in the system enthalpy. Section:1.4
Chapter 1 Biochemistry: An Evolving Science 7 40. If most proteins are found surrounded by water in the cell, what type of functional groups would you expect to find on the surface of a water soluble protein? Ans: Polar and charged amino-acid residues would be present on the surface of the protein. Section: 1.3 41. How are electrostatic forces used in protein folding? Ans: The attraction of two oppositely charged functional groups would be one of the forces helping to form the three-dimensional shape of the protein. Section: 1.3 42. If the First Law of Thermodynamics is true, how can biological processes be carried out? Ans: Although energy cannot be created or destroyed, it can take on different forms, such as heat or chemical energy. Thus, the energy can be stored as chemical bond energy, which can be used to do work. Section: 1.3 43. How can a cell exist if the Second Law of Thermodynamics is true? Ans: Entropy in a local area can be decreased, but only at the expense of increased entropy in the larger area, or universe. Section: 1.3 44. Provide a simple example of entropy processes. Ans: Several examples can be provided, including the random mixture of atoms when two different gases are mixed, or the creation of water molecules from energy gained following the mixture of oxygen and hydrogen under certain conditions. Section: 1.3 45. What does this equation mean: G = H system – TS system < 0? Ans: The free energy change (G) must be negative for a reaction to be spontaneous. Only under these conditions can the total entropy increase. Section: 1.3 46. What is the significance of using G in biochemistry? Ans: Gibbs-free energy, also called the free-energy change, is used to describe the energetics of a reaction. This symbol is used to determine if particular reactions will be spontaneous or biologically feasible. Section: 1.3 47. What thermodynamic and free-energy changes participate in protein folding? Ans: A combination of hydrogen bonds and van der Waals forces affect enthalpy and the entropy associated with hydrophobic interactions. Section: 1.3 48. How do hydrophobic interactions aid in protein folding? Ans: Hydrophobic interaction causes some nonpolar amino acids to aggregate and form the interior of the protein. This results in a net release of heat and a favorable change in the system enthalpy. Section: 1.4

Chapter 1 Biochemistry:An Evolving Science 8 49.What are the enthalpy and entropy changes that accompany the formation of DNA double helixes from complementary single strands of DNA? Ans:There is a loss of entropy from the system because there are fewer degrees of freedom in the double helix as compared to the single strands.Therefore,heat must be released when the two strands combine to form the double helix so as not to violate the Second Law of Thermodynamics. Section 1.3 50.Describe the shape of methane. Ans:Methane is tetrahedral and sp3 hybridized,with bond angles of about 109. Section:Appendix
Chapter 1 Biochemistry: An Evolving Science 8 49. What are the enthalpy and entropy changes that accompany the formation of DNA double helixes from complementary single strands of DNA? Ans: There is a loss of entropy from the system because there are fewer degrees of freedom in the double helix as compared to the single strands. Therefore, heat must be released when the two strands combine to form the double helix so as not to violate the Second Law of Thermodynamics. Section 1.3 50. Describe the shape of methane. Ans: Methane is tetrahedral and sp 3 hybridized, with bond angles of about 109. Section: Appendix