正在加载图片...
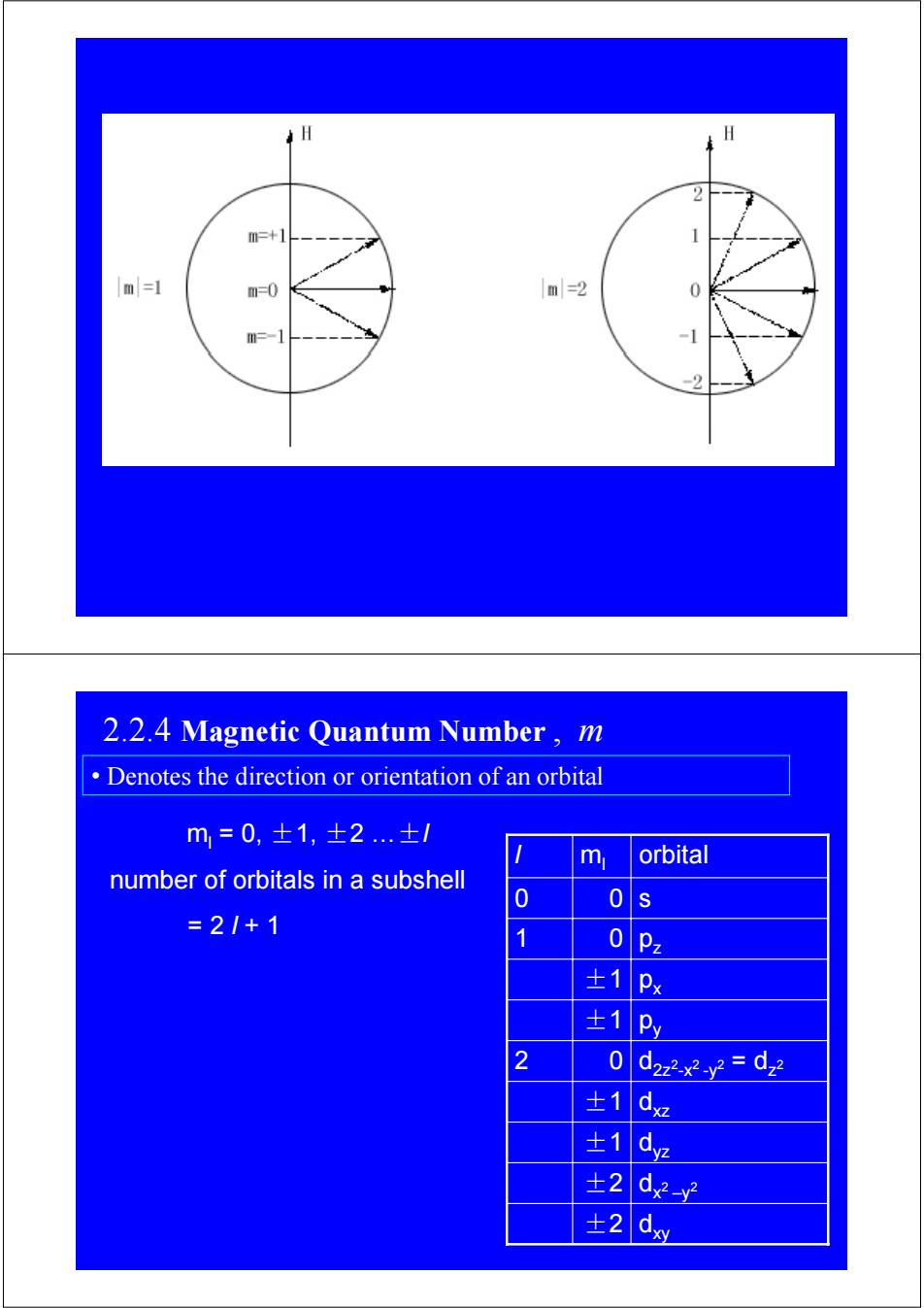
2 m=+1 1 m=1 m=0 m=2 m=-1 2 2.2.4 Magnetic Quantum Number,m Denotes the direction or orientation of an orbital m=0,士1,±2..±/ m orbital number of orbitals in a subshell 0 0 =2/+1 0Pz ±1Px ±1 Py 2 0 d2z2x2y2=dz ±1dz 士1 dye ±2 d-y ±2ml = 0, ±1, ±2 …±l number of orbitals in a subshell = 2 l + 1 ±2 dxy dx2 –y ±2 2 ±1 dyz ±1 0 ±1 ±1 0 0 ml dxz d2z2-x2 -y2 = dz 2 2 py px 1 pz 0 l s orbital 2.2.4 Magnetic Quantum Number , m • Denotes the direction or orientation of an orbital