正在加载图片...
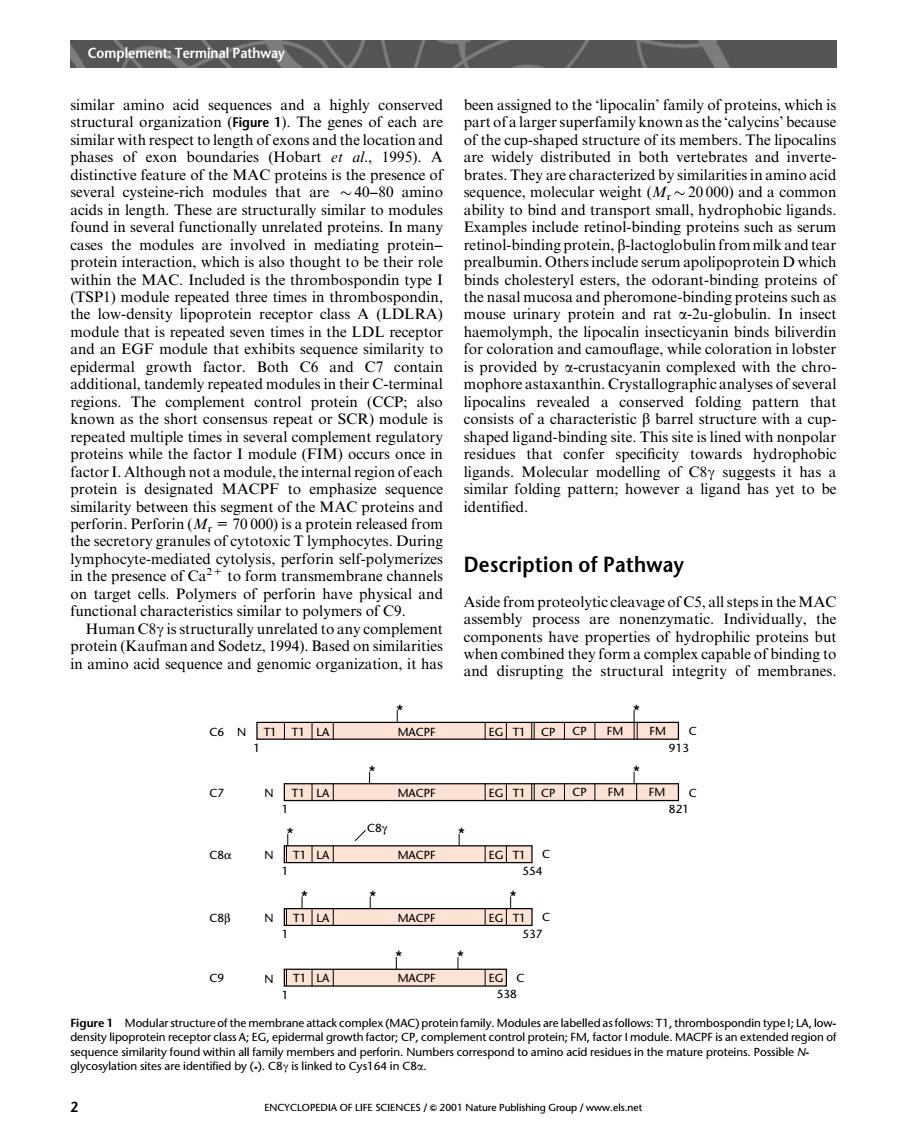
Complement:Terminal Pathway similar to length of Dacn assigned erfamily known as the calv hichse alins phases of exon boundaries (Hobart er al.1995).A are widely distributed in both vertebrates and inverte of brates.They are charact cyste gh) weight(M L TL onally unrelated proteins In ob common Exa roteins such as 二 pre the low-density liponrotein receptor ouse urinary otein and rat module that is repeated seven times in the LDL receptor haemolymph,the lipocalin insecticyanin binds biliverdin and an EGF module that exhibits s equence similarity to lobster oth C7 ent control (CCP als ipocalins revealed a ed folding ern that (FIM ment regulatory Curs confe 01 rein is designated MACPF to er phasize identified ory gran el Description of Pathway target cells.Polymers of pertorn ave physical and functional cha Human C8 y1994 n acid quenc and gnomi rganization,it has and disrupting the structural integrity of membranes. C6 N LA MACPE EG TI CPCP FMFM C 913 EG TICP CP FMFM 821 NT1 LA MACPF EG TI C MACPF 537 Co NTI LA MACPF 338 aneattack cor x(MAC)protein family.Modules relabelledasfollows:T1,thrombospondin typel:LA,low n o by(-). Cy me C82 ENCYCLOPEDIA OF LIFE SCIENCES/2001 Nature Publishing Group /www.els.net similar amino acid sequences and a highly conserved structural organization (Figure 1). The genes of each are similar with respect to length of exons and the location and phases of exon boundaries (Hobart et al., 1995). A distinctive feature of the MACproteins is the presence of several cysteine-rich modules that are 40–80 amino acids in length. These are structurally similar to modules found in several functionally unrelated proteins. In many cases the modules are involved in mediating protein– protein interaction, which is also thought to be their role within the MAC. Included is the thrombospondin type I (TSP1) module repeated three times in thrombospondin, the low-density lipoprotein receptor class A (LDLRA) module that is repeated seven times in the LDL receptor and an EGF module that exhibits sequence similarity to epidermal growth factor. Both C6 and C7 contain additional, tandemly repeated modules in their C-terminal regions. The complement control protein (CCP; also known as the short consensus repeat or SCR) module is repeated multiple times in several complement regulatory proteins while the factor I module (FIM) occurs once in factor I. Although not a module, the internal region of each protein is designated MACPF to emphasize sequence similarity between this segment of the MACproteins and perforin. Perforin (Mr= 70 000) is a protein released from the secretory granules of cytotoxic T lymphocytes. During lymphocyte-mediated cytolysis, perforin self-polymerizes in the presence of Ca2+ to form transmembrane channels on target cells. Polymers of perforin have physical and functional characteristics similar to polymers of C9. Human C8g is structurally unrelated to any complement protein (Kaufman and Sodetz, 1994). Based on similarities in amino acid sequence and genomic organization, it has been assigned to the ‘lipocalin’ family of proteins, which is part of a larger superfamily known as the ‘calycins’ because of the cup-shaped structure of its members. The lipocalins are widely distributed in both vertebrates and invertebrates. They are characterized by similarities in amino acid sequence, molecular weight (Mr 20 000) and a common ability to bind and transport small, hydrophobic ligands. Examples include retinol-binding proteins such as serum retinol-binding protein, b-lactoglobulin from milk and tear prealbumin. Others include serum apolipoprotein D which binds cholesteryl esters, the odorant-binding proteins of the nasal mucosa and pheromone-binding proteins such as mouse urinary protein and rat a-2u-globulin. In insect haemolymph, the lipocalin insecticyanin binds biliverdin for coloration and camouflage, while coloration in lobster is provided by a-crustacyanin complexed with the chromophore astaxanthin. Crystallographic analyses of several lipocalins revealed a conserved folding pattern that consists of a characteristic b barrel structure with a cupshaped ligand-binding site. This site is lined with nonpolar residues that confer specificity towards hydrophobic ligands. Molecular modelling of C8g suggests it has a similar folding pattern; however a ligand has yet to be identified. Description of Pathway Aside from proteolytic cleavage of C5, all steps in theMAC assembly process are nonenzymatic. Individually, the components have properties of hydrophilic proteins but when combined they form a complex capable of binding to and disrupting the structural integrity of membranes. * * C6 N T1 T1 LA MACPF EG T1 CP CP FM FM * * 913 C C7 N T1 LA MACPF EG T1 CP CP FM FM * * 821 C 1 1 C8α N T1 LA MACPF EG T1 * 554 C 1 C8γ * C8β N T1 LA MACPF EG T1 537 C 1 * * * C9 N T1 LA MACPF EG 538 C 1 Figure 1 Modular structure of the membrane attack complex (MAC) protein family. Modules are labelled as follows: T1, thrombospondin type I; LA, lowdensity lipoprotein receptor class A; EG, epidermal growth factor; CP, complement control protein; FM, factor I module. MACPF is an extended region of sequence similarity found within all family members and perforin. Numbers correspond to amino acid residues in the mature proteins. Possible Nglycosylation sites are identified by (*). C8g is linked to Cys164 in C8a. Complement: Terminal Pathway 2 ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net