正在加载图片...
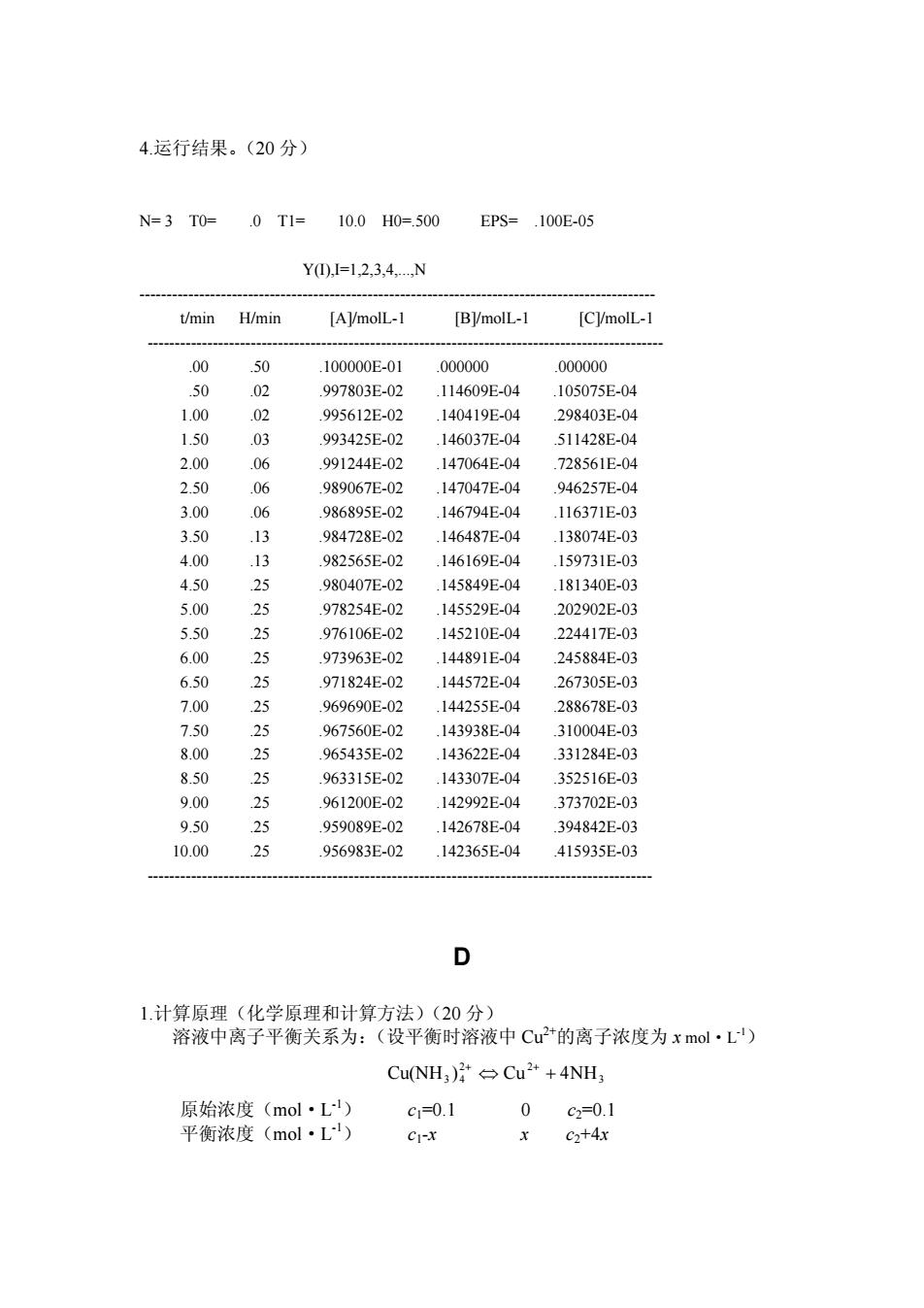
4.运行结果。(20分) N=3T0= 0T1= 10.0H0=500 EPS=.100E-05 Y0,=1,2,3.,4N t/min H/min [AVmolL-1 [B]/molL- [CVmolL- .50 100000E-01 000000 000000 50 03 0g7803F.02 ,114609E-04 105075E-.04 1.0 02 095612.02 .140419E-04 298403E-04 993425E.02 146037E-4 511428E-04 2.00 06 991244E-0 .147064E-04 .728561E-0 2.50 06 989067E-02 .147047E-04 946257E-04 3.00 06 98695F.02 .146794E-04 .116371E-03 3.50 984728E-02 146487E-04 138074E.03 982565E-02 25 46169E-0 159731E-03 980407E-4 .14849上-0 181340E-0 5.00 25 978254E-02 145529E-04 202902E-03 5.50 5 976106E-02 .145210E-04 224417E-03 6.00 973963E-02 .144891E-04 245884E-03 971824E-02 1445T2E 267305E-03 25 969690E-( 14425E- 288678 150 967560E-0 .143938E-04 .310004E-03 8. 965435E-01 .143622E-04 331284E-03 .50 25 963315F-02 143307E04 352516F-03 9.00 961200E-02 142992E.04 373702E-03 5 959089 5 142678E-04 394842E-03 956983 .142365E-04 .415935E-0 1计算原理(化学原理和计算方法)(20分) 溶液中离子平衡关系为: (设平衡时溶液中Cu2+的离子浓度为xmol·L) Cu(NH:)Cu+4NH3 原始浓度(mol·L) c,=0.1 0c=0.1 平衡浓度(mol·L) CI-X c+4x4.运行结果。(20 分) N= 3 T0= .0 T1= 10.0 H0=.500 EPS= .100E-05 Y(I),I=1,2,3,4,...,N ----------------------------------------------------------------------------------------------- t/min H/min [A]/molL-1 [B]/molL-1 [C]/molL-1 ----------------------------------------------------------------------------------------------- .00 .50 .100000E-01 .000000 .000000 .50 .02 .997803E-02 .114609E-04 .105075E-04 1.00 .02 .995612E-02 .140419E-04 .298403E-04 1.50 .03 .993425E-02 .146037E-04 .511428E-04 2.00 .06 .991244E-02 .147064E-04 .728561E-04 2.50 .06 .989067E-02 .147047E-04 .946257E-04 3.00 .06 .986895E-02 .146794E-04 .116371E-03 3.50 .13 .984728E-02 .146487E-04 .138074E-03 4.00 .13 .982565E-02 .146169E-04 .159731E-03 4.50 .25 .980407E-02 .145849E-04 .181340E-03 5.00 .25 .978254E-02 .145529E-04 .202902E-03 5.50 .25 .976106E-02 .145210E-04 .224417E-03 6.00 .25 .973963E-02 .144891E-04 .245884E-03 6.50 .25 .971824E-02 .144572E-04 .267305E-03 7.00 .25 .969690E-02 .144255E-04 .288678E-03 7.50 .25 .967560E-02 .143938E-04 .310004E-03 8.00 .25 .965435E-02 .143622E-04 .331284E-03 8.50 .25 .963315E-02 .143307E-04 .352516E-03 9.00 .25 .961200E-02 .142992E-04 .373702E-03 9.50 .25 .959089E-02 .142678E-04 .394842E-03 10.00 .25 .956983E-02 .142365E-04 .415935E-03 --------------------------------------------------------------------------------------------- D 1.计算原理(化学原理和计算方法)(20 分) 溶液中离子平衡关系为:(设平衡时溶液中 Cu2+的离子浓度为 x mol·L-1) 3 2 2 Cu(NH3 ) 4 ⇔ Cu + 4NH + + 原始浓度(mol·L-1) c1=0.1 0 c2=0.1 平衡浓度(mol·L-1) c1-x x c2+4x