正在加载图片...
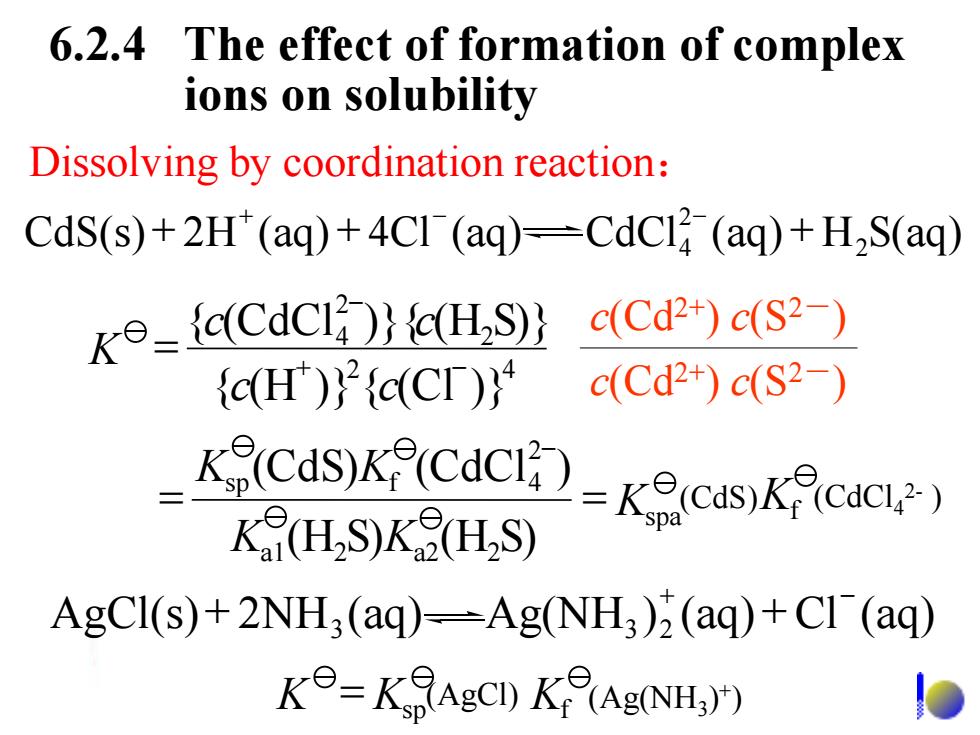
6.2.4 The effect of formation of complex ions on solubility Dissolving by coordination reaction: CdS(s)+2H(aq)+4CI (aq)=CdCl(aq)+H2S(aq) K⊙-CdCi)}cH,S} c(Cd2+)c(S2-) {cH)}2{c(Cr)} c(Cd2+)c(S2-) 2对- AgCl(s)+2NH3(aq)-Ag(NH3)2(aq)+Cl (aq) K=KAgCD K(Ag(NH 6.2.4 The effect of formation of complex ions on solubility c(Cd2+) c(S2-) c(Cd2+) c(S2-) CdS(s) 2H (aq) 4Cl (aq) CdCl (aq) H S(aq) 2 2 4 + + + + - - 2 4 2 2 4 { (H )} { (Cl )} { (CdCl )}{ (H S)} c c c c K = + - - AgCl(s) 2NH (aq) Ag(NH ) (aq) Cl (aq) 3 3 2 + - + + f spa a1 2 a2 2 2 sp f 4 (H S) (H S) (CdS) (CdCl ) K K K K K K = = - (CdS) (CdCl4 2- ) K Ksp Kf = (AgCl) (Ag(NH3 ) + ) Dissolving by coordination reaction: