正在加载图片...
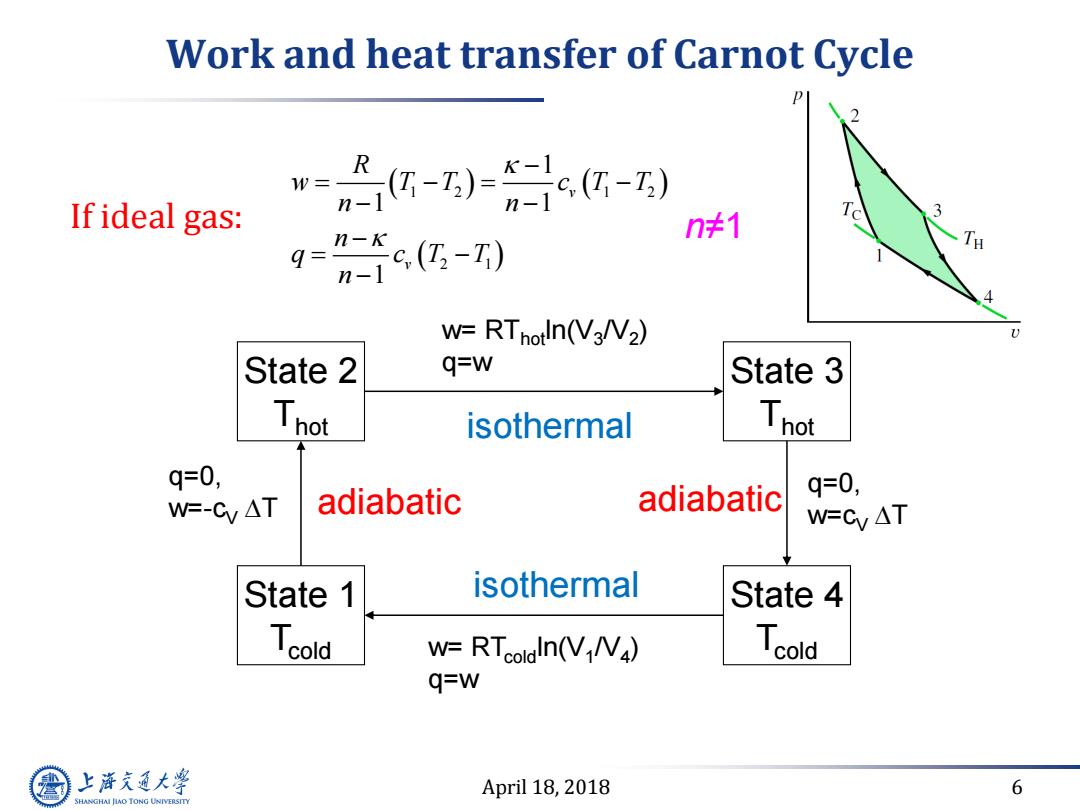
Work and heat transfer of Carnot Cycle w= If ideal gas: -)0c-) n-1 gg-) n≠1 W=RTnotln(V3NV2) State 2 q=w State 3 Thot isothermal Thot q=0, adiabatic adiabatic q=0, W=-Cy AT W=Cy AT State 1 isothermal State 4 Tcold W=RTcoldln(VN) Tcold q=w 上游充通大 April 18,2018 6 SHANGHAI JIAO TONG UNIVERSITYApril 18, 2018 6 Work and heat transfer of Carnot Cycle State 2 Thot State 3 Thot State 1 Tcold State 4 Tcold isothermal adiabatic adiabatic isothermal q=0, w=-cV T q=0, w=cV T w= RThotln(V3 /V2 ) q=w w= RTcoldln(V1 /V4 ) q=w If ideal gas: 1 2 1 2 2 1 1 1 1 1 v v R w T T c T T n n n q c T T n n≠1