正在加载图片...
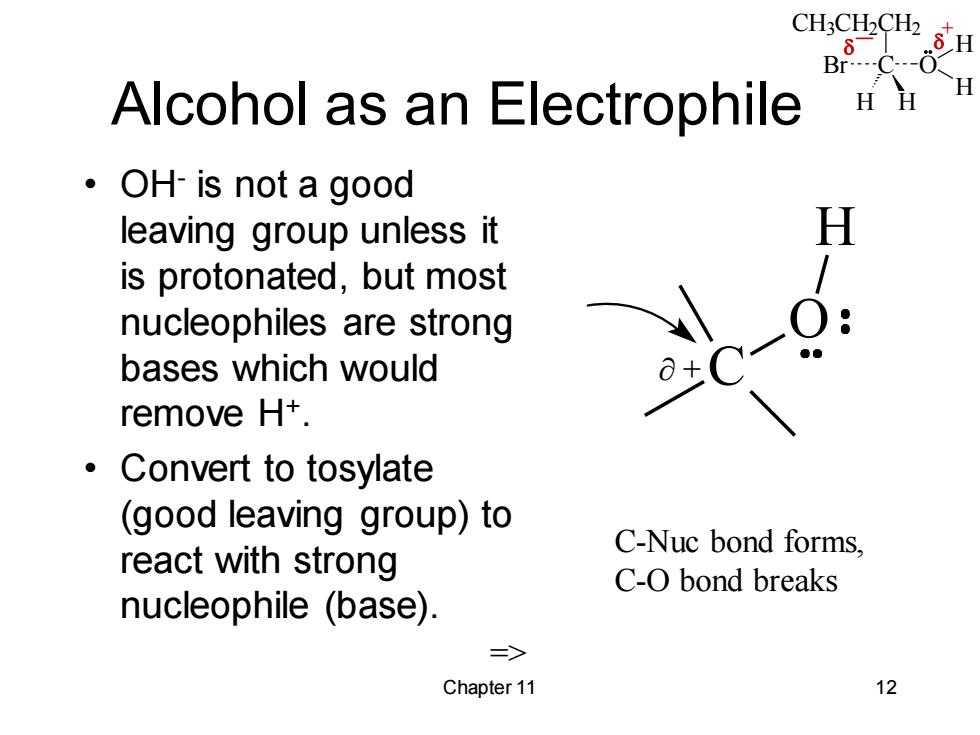
CH3CH2CH2 6 H Br Alcohol as an Electrophile OH-is not a good leaving group unless it is protonated,but most nucleophiles are strong bases which would remove H+. ·Convert to tosylate (good leaving group)to react with strong C-Nuc bond forms, C-O bond breaks nucleophile (base). => Chapter 11 12 CH3CH2CH2 C H H Br O H H _ + Chapter 11 12 Alcohol as an Electrophile • OHis not a good leaving group unless it is protonated, but most nucleophiles are strong bases which would remove H+ . • Convert to tosylate (good leaving group) to react with strong nucleophile (base). => C O H + C-Nuc bond forms, C-O bond breaks