正在加载图片...
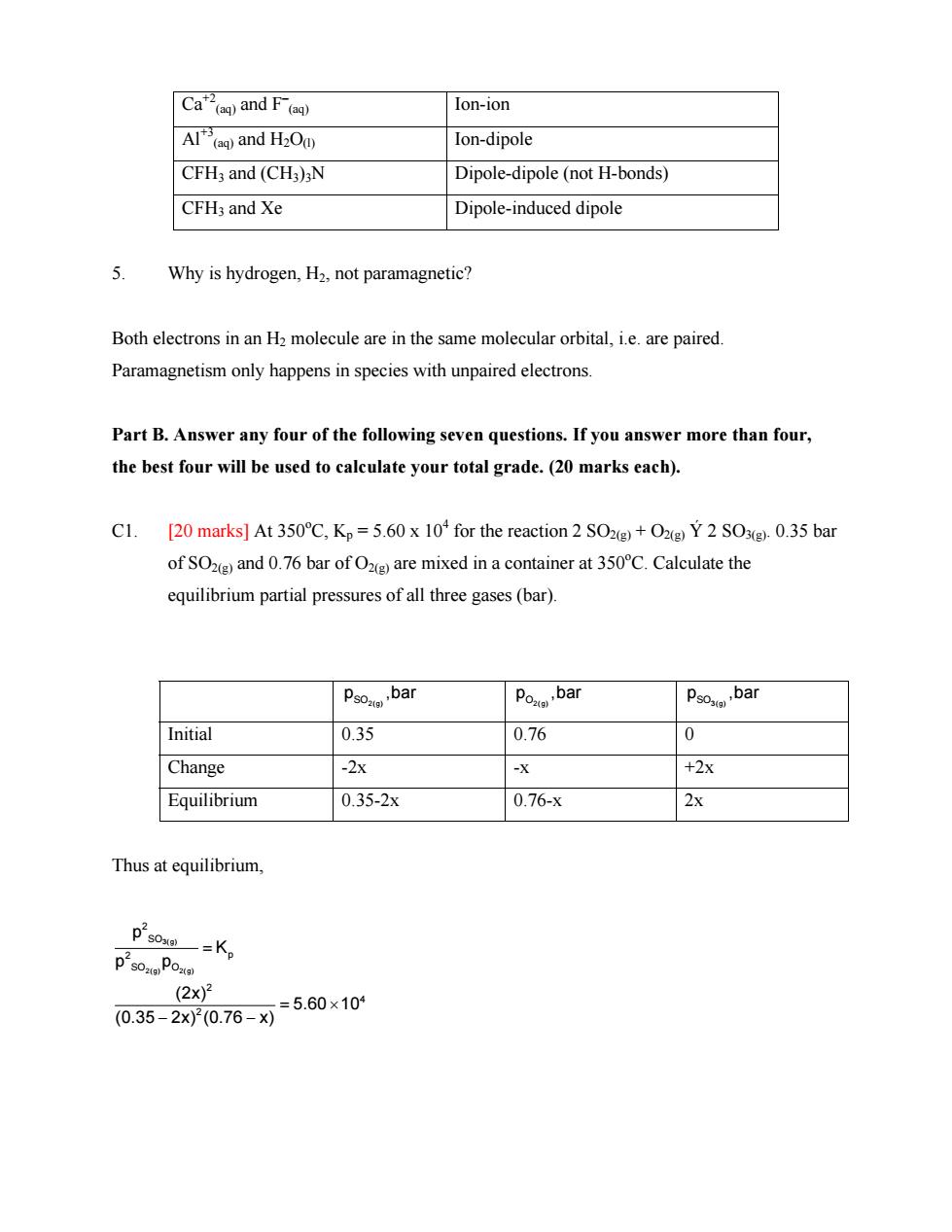
Ca”and F(e Ion-ion Al()and H2Od) Ion-dipole CFH3 and (CH3)3N Dipole-dipole(not H-bonds) CFH3 and Xe Dipole-induced dipole 5 Why is hydrogen,H2.not paramagnetic? Both electrons in an H2 molecule are in the same molecular orbital,i.e.are paired Paramagnetism only happens in species with unpaired electrons Part B.Answer any four of the following seven questions.If you answer more than four, the best four will be used to calculate your total grade.(20 marks each). [20 marks]At 350C.Kp=5.60 x 10 for the reaction 2 SO2+O Y 2 SOx(g).0.35 bar of SO(and 0.76 bar ofO are mixed in a container at 350C.Calculate the equilibrium partial pressures of all three gases(bar). Initial 0.35 0.76 0 Change -2x -X +2X Equilibrium 0.35-2x 0.76-x 2x Thus at equilibrium, ps0一=Kp ps0Pom (2x)2 0.35-2x00.76-X=5.60×10Ca+2 (aq) and F– (aq) Ion-ion Al+3 (aq) and H2O(l) Ion-dipole CFH3 and (CH3)3N Dipole-dipole (not H-bonds) CFH3 and Xe Dipole-induced dipole 5. Why is hydrogen, H2, not paramagnetic? Both electrons in an H2 molecule are in the same molecular orbital, i.e. are paired. Paramagnetism only happens in species with unpaired electrons. Part B. Answer any four of the following seven questions. If you answer more than four, the best four will be used to calculate your total grade. (20 marks each). C1. [20 marks] At 350o C, Kp = 5.60 x 104 for the reaction 2 SO2(g) + O2(g) Ý 2 SO3(g). 0.35 bar of SO2(g) and 0.76 bar of O2(g) are mixed in a container at 350o C. Calculate the equilibrium partial pressures of all three gases (bar). SO2(g) p ,bar O2(g) p ,bar SO3(g) p ,bar Initial 0.35 0.76 0 Change -2x -x +2x Equilibrium 0.35-2x 0.76-x 2x Thus at equilibrium, 3(g) 2(g) 2(g) 2 SO 2 p SO O 2 4 2 p K p p (2x) 5.60 10 (0.35 2x) (0.76 x) = = × − −