正在加载图片...
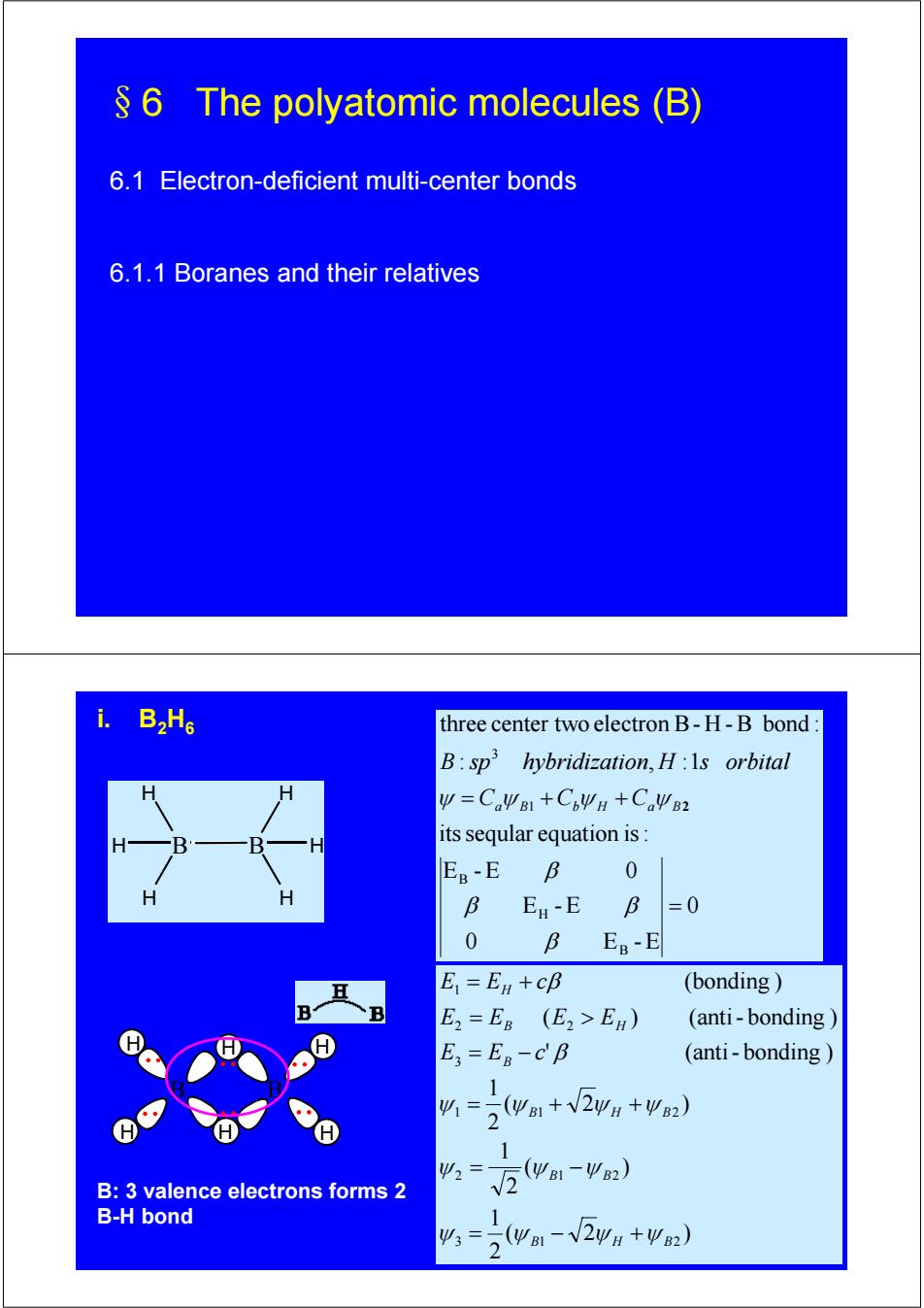
6 The polyatomic molecules (B) 6.1 Electron-deficient multi-center bonds 6.1.1 Boranes and their relatives i. B2H6 three center two electron B-H-B bond: B:sp hybridization,H:Is orbital y=CaWB1+C6ΨH+CaW2 its seqular equation is: B EB-E B 0 H B E-E B =0 0 B EB-E E=En +cB (bonding E2=ER (E2>EB) (anti-bonding E=ER-CB (anti-bonding 1 41=。(wB1+√2ΨH+ΨB2) 2 1 Ψ2 B:3 valence electrons forms 2 V2wa1-2) B-H bond 1 43=5(yB1-√2wH+Ψ2) 2§6 The polyatomic molecules (B) 6.1 Electron-deficient multi-center bonds 6.1.1 Boranes and their relatives B H H H B H H H 0 0 E -E E -E E -E 0 itsseqular equation is: : , :1 three center two electron B- H -B bond : B H B 1 3 = = + + β β β β ψ Ca ψ B Cb ψ H Ca ψ B2 B sp hybridization H s orbital i. B2H6 B H H H H H H B B: 3 valence electrons forms 2 B-H bond ( 2 ) 2 1 ( ) 2 1 ( 2 ) 2 1 ' (anti- bonding ) ( ) (anti- bonding ) (bonding ) 3 1 2 2 1 2 1 1 2 3 2 2 1 B H B B B B H B B B H H E E c E E E E E E c ψ ψ ψ ψ ψ ψ ψ ψ ψ ψ ψ β β = − + = − = + + = − = > = +