正在加载图片...
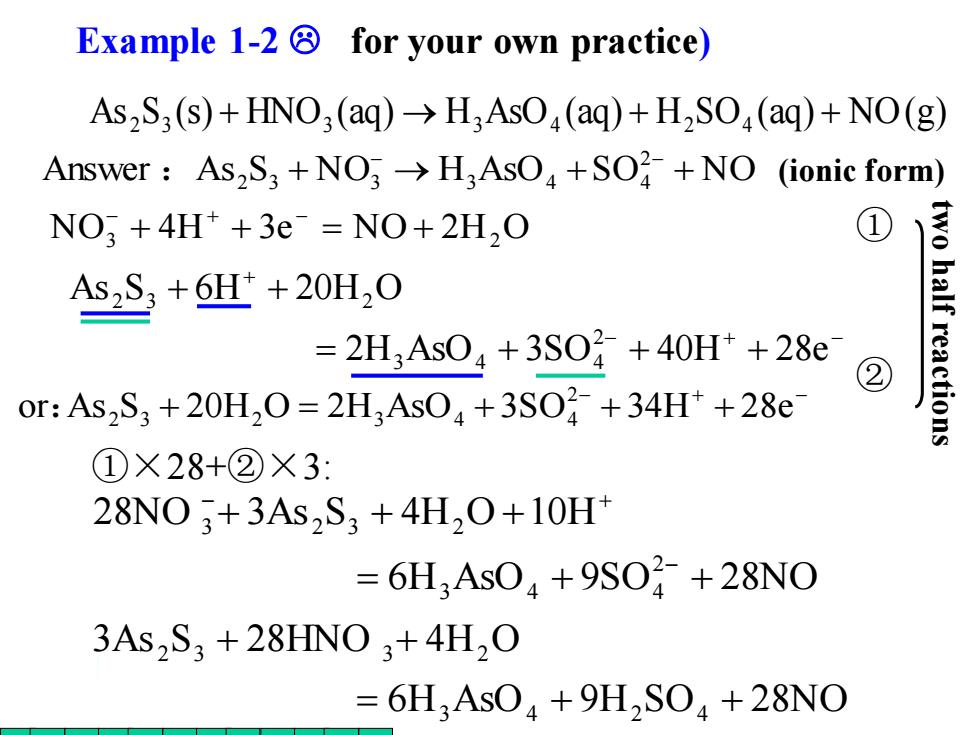
Example 1-2 for your own practice) As2S3 (s)+HNO3 (aq)>H3AsO(aq)+H2SO(aq)+NO(g) Answer As2S3 +NO3>H;AsO+SO2+NO (ionic form) NO:+4H*+3e=NO+2H,O ① As2S+6H+20H20 =2H3As04+3S0+40H++28e ② or:As2S3+20H20=2H3As04+3S0¥+34H+28e ctions ①×28+②×3: 28NO3+3As2S,+4H20+10H =6H,As04+9SO2+28NO 3As2S3+28HN03+4H2O =6H3AsO4+9H2S04+28NO As S (s) HNO (aq) H AsO (aq) H SO (aq) NO(g) 2 3 + 3 → 3 4 + 2 4 + ①×28+②×3: ② ① Example 1-2 for your own practice) 6H AsO 9H SO 28NO 3As S 28HNO 4H O 3 4 2 4 2 3 3 2 = + + + + 6H AsO 9SO 28NO 28NO 3As S 4H O 10H 2 3 4 4 3 2 3 2 = + + + + + − − + − + − or As S + 20H O = 2H AsO + 3SO + 34H + 28e 2 : 2 3 2 3 4 4 − + − + = + + + + + 2H AsO 3SO 40H 28e As S 6H 20H O 2 3 4 4 2 3 2 NO3 + 4H + 3e = NO + 2H2 O − + − Answer As S N O H AsO SO N O 2 2 3 + 3 → 3 4 + 4 + : − − (ionic form)two half reactions