正在加载图片...
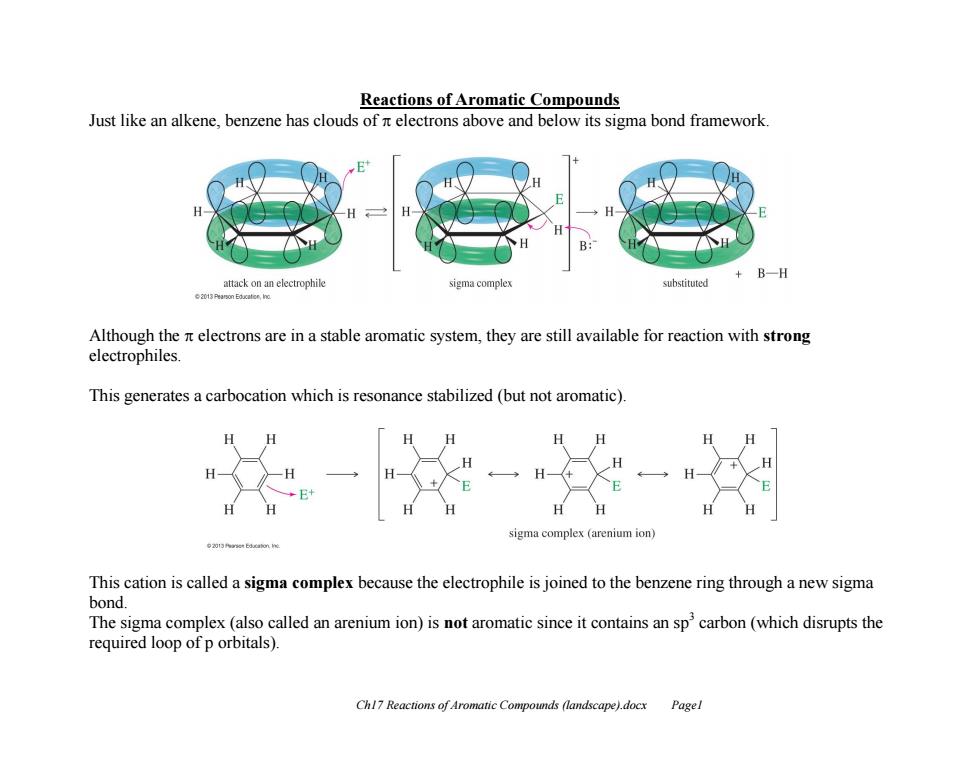
Reactions of Aromatic Compounds Just like an alkene,benzene has clouds of it electrons above and below its sigma bond framework B-H attack on an electrophile sigma complex substituted 20PronEae,Ino Although the nt electrons are in a stable aromatic system,they are still available for reaction with strong electrophiles This generates a carbocation which is resonance stabilized(but not aromatic) igma comple (arenium ion) This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex(also called an arenium ion)is not aromatic since it contains an sp'carbon (which disrupts the required loop of p orbitals). Chl7 Reactions of Aromatic Compounds (landscape).docx PagelCh17 Reactions of Aromatic Compounds (landscape).docx Page1 Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals)