正在加载图片...
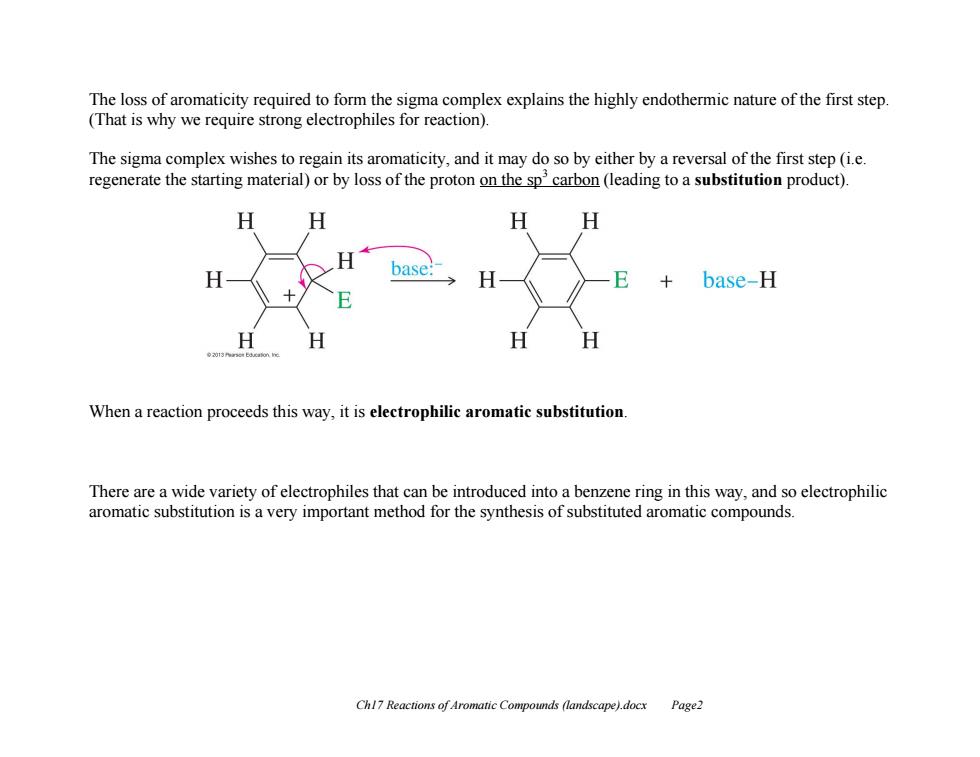
The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity,and it may do so by either by a reversal of the first step(i.e. regenerate the starting material)or by loss of the proton on the spcarbon(leading to a substitution product). H H H base E base-H H H When a reaction proceeds this way,it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way,and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Chl7 Reactions of Aromatic Compounds (landscape).docx Page2 Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either by a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds