正在加载图片...
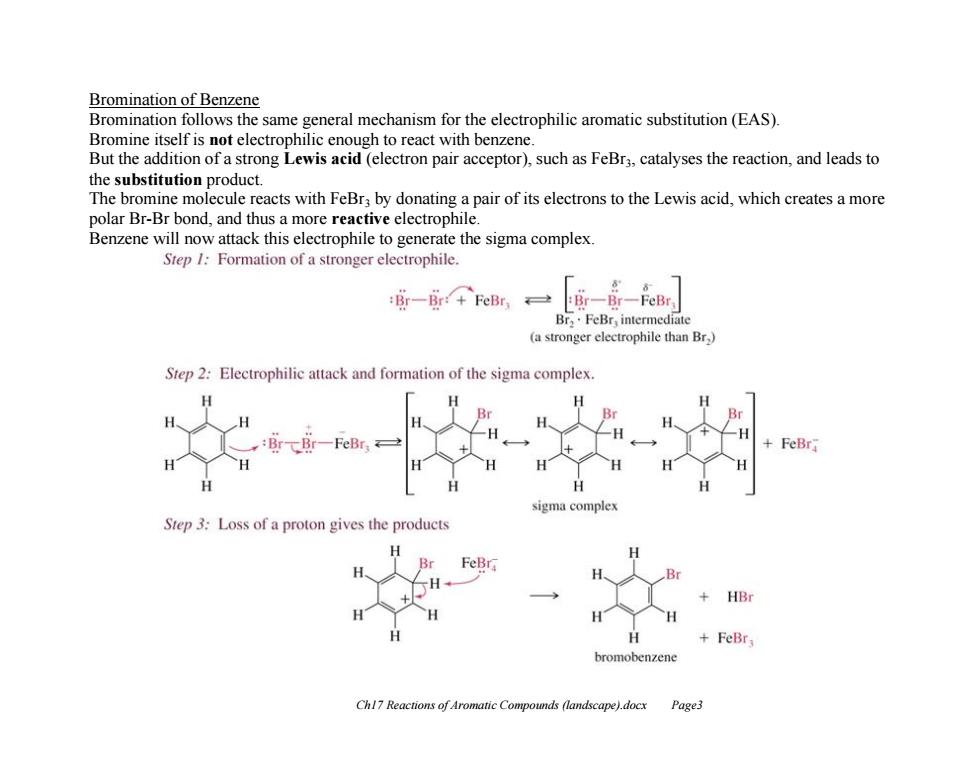
Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution(EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid(electron pair acceptor),such as FeBr3,catalyses the reaction,and leads to the substitution product. The bromine molecule reacts with FeBr;by donating a pair of its electrons to the Lewis acid,which creates a more polar Br-Br bond,and thus a more reactive electrophile. Benzene will now attack this electrophile to generate the sigma complex Step /Formation of a stronger electrophile. Br-Bir:+FeBry Br,.FeBr,intermediate (a stronger electrophile than Br) Step 2:Electrophilic attack and formation of the sigma complex. FeBr ma complex Step 3:Loss of a proton gives the products FeBr, bromobenzene Chl7 Reactions of Aromatic Compounds (landscape).docx Page3Ch17 Reactions of Aromatic Compounds (landscape).docx Page3 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. The bromine molecule reacts with FeBr3 by donating a pair of its electrons to the Lewis acid, which creates a more polar Br-Br bond, and thus a more reactive electrophile. Benzene will now attack this electrophile to generate the sigma complex