正在加载图片...
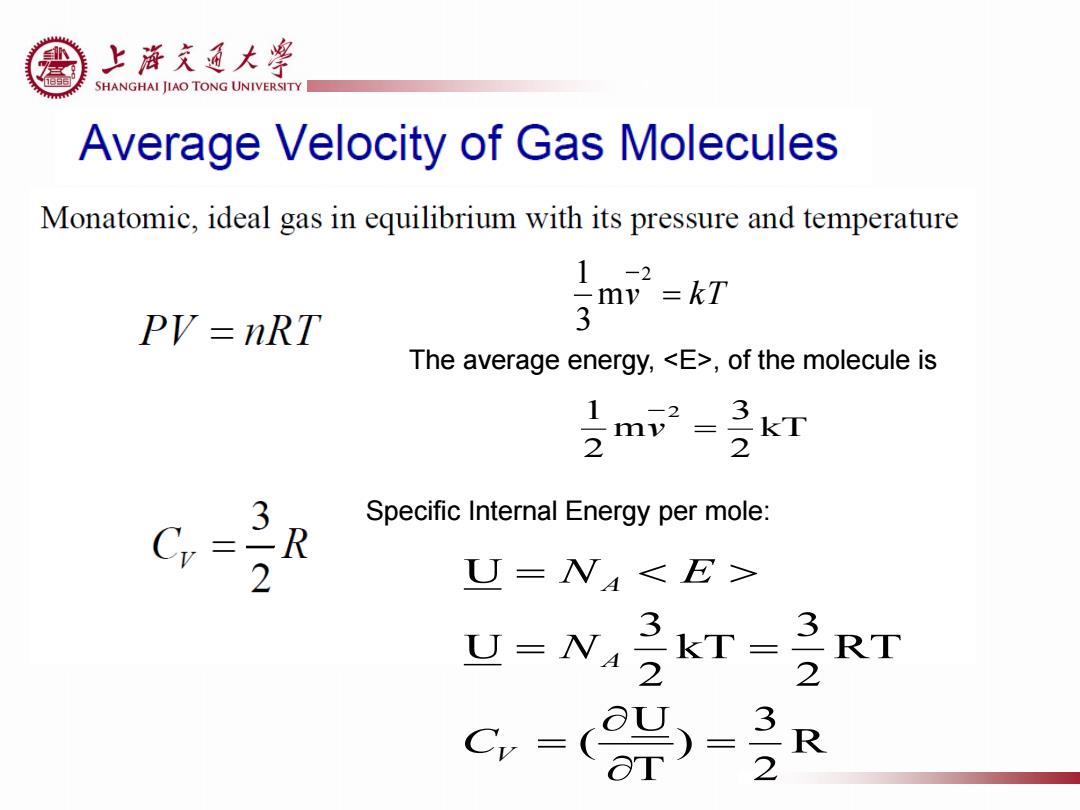
上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY Average Velocity of Gas Molecules Monatomic,ideal gas in equilibrium with its pressure and temperature 1-2 my=kT PV=nRT 3 The average energy,<E>,of the molecule is 2 3 Specific Internal Energy per mole: R 2 U=N<E> U=N 3 kT= 32 RT C-( 3-2 Rv kT 2 m 3 1 The average energy, <E>, of the molecule is kT 2 3 m 2 1 2 v R 2 3 ) T U ( RT 2 3 kT 2 3 U U V A A C N N E Specific Internal Energy per mole: